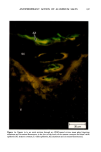
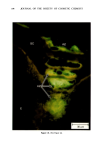
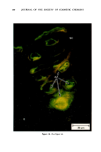
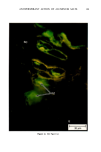
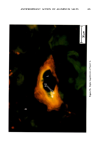
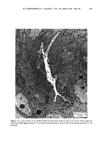
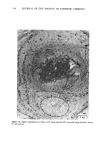
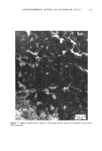
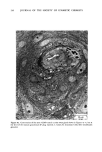
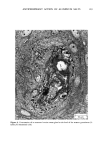
ANTIPERSPIRANT ACTION OF ALUMINUM SALTS 221 viable epidermis but were commonly found only at a very superficial level, in the sweat gland ostium. Histologically, from the standpoint of fluorescence microscopy studies, and particularly from those of TEM, these occlusive masses strongly resembled those which had been seen when the perspiration-inhibiting agent was ACH. However, the ultrastructure studies did not show occlusive masses as extensive as those which were demonstrated for ACH-inhibited glands, suggesting that the plug was very superficial. (An alternative is that the physical structure of an AZAP plug is more readily destroyed by the procedures used to prepare the tissue for TEM sectioning). From these observations, it is concluded that the mechanism of action by which AZAP inhibits eccrine sweat gland function is quite similar to that which has been described for ACH, namely that the antiperspirant forms a plug within the sweat duct that blocks the flow of sweat. Both tape-stripping studies and ultrastructure microscopy have suggested that the location of this plug is relatively more superficial in the duct than is that of ACH, but that it can be as deep as 80-100/.tm in some instances. The mechanism of poral occlusion, perhaps by formation of a hydroxide gel as suggested by others (3), is now established for AZAP. However, these studies have not lent additional insight into one apparent paradox. TEM studies have shown the AZAP plug's presence to be very near the skin surface. However, it has been shown that AZAP's duration of antiperspirant activity is at least as long as four weeks (3), and our own studies (unpublished observations) indicate that ACH's activity disappears after about two weeks. If these observations are correct, then they would suggest that the structural integrity of the AZAP plug is greater than that of ACH. V. CONCLUSIONS Morin-fluorescence microscopy of AZAP-inhibited eccrine sweat glands demon- strated the presence of aluminum and zirconium in the duct in quantities sufficient enough to occlude the flow of sweat. The metals were found mainly in the intracorneal duct but frequently in the intraepidermal duct as well. Transmission electron microscopy showed occlusive masses at similar locations within the duct. AZAP's mechanism of action is similar to ACH's in that it forms a plug but the location of the AZAP plug is probably more superficial. VI. ACKNOWLEDGEMENT We are indebted to Morris Shelanski, M.D. of Products Investigations, Inc., Consho- hocken, Pa. for performing the biopsy procedures. REFERENCES (1) R. P. Quatrale, A. H. Waldman, J. G. Rogers and C. B. Felger, The mechanism of antiperspirant action by aluminum salts. I. The effect of cellophane tape stripping on aluminum salt-inhibited eccrine sweat glands,J. Soc. Cosmet. Chem., 32, 67-73 (1981). (2) R. P. Quatrale, D. W. Coble, K. L. Stoner and C. B. Felger, The mechanism of antiperspirant action by aluminum salts. II. Histological observations of human eccrine sweat glands inhibited by aluminum chlorohydrate,J. Soc. Cosmet. Chem., 32, 107-136 (1981).
J. Soc. Cosmet. Chem., 32, 223-229 (July/August 1981) Percutaneous penetration of hair dyes HOWARD I. MAIBACH, Department of Dermatology, School of Medicine, University of California, San Francisco, CA 94143 and LESZEKJ. [VOLFRAM, Clairol Inc., Stamford, CT 06922 Received May 21, 1981. Synopsis Scalp penetration of diaminoanisole (DAA), p-phenylenediamine (PPD), and N4,N4-Bis-(2-hydroxyethyl)- N'-methyl-2-nitro-p-phenylenediamine (HC Blue #1) that occurs under conditions of hair dye usage was evaluated for both Rhesus monkey and man using •4C labeled materials. Both species showed a remarkably similar pattern of dye penetration. Thus the average dose excretion of DAA in the Rhesus monkey was 0.02% and in man 0.015% PPD excretion in monkey and man was 0.14% and HC Blue #1 excretion in the monkey was 0.12% and in man, 0.09%. INTRODUCTION Hair dyes have been in use for several decades yet even recent studies of their skin penetration potential have been restricted primarily to their evaluation in rats and dogs (1-4). Although useful, these results are difficult to relate to man and thus the uncertainty as to •the extent of penetration under practical use conditions of the fully formulated products continues. Kiese and Rausher (5) studied the absorbtion of p-toluenediamine in human skin but their analytical approach, based on the excretion in vivo of N,N'-diacetyl-p-toluenediamine, did not allow for conclusive quantification of the penetration data. Qualitative studies in man suggested that penetration occurred but could offer no quantitation (6). Subsequent investigation raised the question of whether thin layer chromatographic data were real or artifacts (7). We have conducted investigations into the percutaneous absorbtion in vivo of three commonly used hair dye ingredients in rhesus monkey and man. The methodology was patterned after the procedure developed by Feldmann and Maibach (8) for measurement of percutaneous absorbtion in man. This method involves quantifying absorbtion on the basis of the percent of radiactivity excreted in the urine for at least five days following application of a known amount of the labeled compound. EXPERIMENTAL 1. DYE PREPARATION. Commercially available hair dye products were labeled with radioactive materials (ICN Pharmaceuticals, Inc., Irvine, CA). Oxidative hair colors were enriched as follows: 223
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)