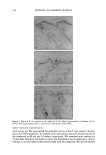
154 JOURNAL OF COSMETIC SCIENCE 29 • 27 .• 25 n, 23 E = 2'1 o '19 ß 'v 17 5 I I I I I I I I I I I I I I I I I I I I I I I I 0 10 20 30 40 50 Concentration of Miceliar SDS (mM) Figure 4. Measured effective hydrodynamic radii of SDS/C•2E 6 mixed micelies for o• m = 1 (0), o• m = 0.83 ([•), and o•,• = 0.50 (l•) as a function of the concentration of miceliar SDS (that is, the SDS concentration minus the predicted SDS toohomer concentration oqC• see Tables I and II) (30,31) using DLS at 25øC in 0.1 M NaC1. The miceliar radii were determined using a CONTIN analysis. The error bars reflect a 95% confidence interval based on eight samples at each SDS concentration. The actual hydrodynamic radius is equal to the intercept. Table III The Micelie Hydrodynamic Radii Determined Using a CONTIN Analysis of the Correlation Function O• s R H 1 20+1 0.83 24 + 1 0.50 27 + 3 The actual hydrodynamic radius of the micelie is determined by extrapolating the effective hydrodynamic radii in Figure 3 to a zero micelie concentration. The error values reflect a 95% confidence interval. model is based on the premise that only micelles that are small enough to access the aqueous pores in the SC can contribute to surfactant penetration into the epidermis. Other researchers have determined the average aqueous pore radius in the skin using permeability and/or conductivity measurements in the context of hindered-transport theories, and have reported radii values between 10 • and 28 • (9,12,43-45). Based on the micelie hydrodynamic radii reported in Table III and a purely steric model of micelie penetration into the skin that ignores electrostatic interactions (discussed below), and considering a skin aqueous pore radius of at most 28 •, the OQn = 1 micelles should be able to penetrate into the SC more easily than the OQn = 0.83 and the o•n• = 0.50 micelies. This conclusion is consistent with the results of the multiple linear regression
PENETRATION OF MIXED MICELLES INTO THE EPIDERMIS 155 analysis presented above, and lends greater validity tO the idea put forward by us recently (28) that steric factors can play a key role in determining whether the miceliar surfactant can penetrate into the skin. Concerns that the penetration of SDS into the SC may alter the characteristic pore size in the SC are mitigated by the work of Peck et aL (9). These authors found that the average pore size of the SC measured by hindered transport was unaffected by exposing the epidermis to SDS solutions for 18 hours. Instead, they concluded that the increased permeability of the skin resulted from an increase in the effective porosity/tortuosity of the SC. Nevertheless, we believe that additional research should be conducted to better understand the effect of surfactant penetration into the skin on the aqueous pathways of the SC. POSSIBLE ELECTROSTATIC EFFECTS ON SDS SKIN PENETRATION Interestingly, the oL m = i micelies have an equal, or slightly lower value, of the regres- sion coefficient, b (0.032 + 0.014), than the one reported in our recent paper (0.043 + 0.006) (28), while the SDS monomers penetrate into the epidermis much more readily according to the results reported in this paper (a = 4.1 + 1.0 here versus a = 0.14 + 0.04 in reference (28)). The main difference in the conditions corresponding to the two sets of experiments is the presence of 0.1 M NaC1 in the systems examined in this paper, compared to the no-added-salt case considered in the previous paper (28). It is known that the skin carries a net negative charge (9), and that the addition of salt screens this negative charge. Screening the negative charge would make it easier for negatively charged SDS monomers to approach the skin surface, which could explain the observed increase in the value of a. However, the same argument should apply to the O• m = 1 micelies, which are also negatively charged. Nevertheless, the SDS micelies do not show a significant change in their contribution to SDS penetration upon the addition of salt. In fact, the pure SDS micelies appear to be somewhat less able to contribute to Cs•i, in the presence of salt (b = 0.0032) than in the absence of salt (b -- 0.0043 in reference (28)). It is important to keep in mind, however, that the addition of salt may lead to some micelie growth (32,46). As a result, applying our model of micelie penetration, the larger micelies in the presence of salt may be less able to penetrate into the skin, thus counteracting the effect of any decrease in the electrostatic repulsions between the skin and the SDS micelies. The discussion above about potential electrostatic effects affecting surfactant penetration into the skin indicates that steric hindrance may not be the only factor determining whether a micelie can penetrate into the aqueous pores of the skin. Iontophoresis experiments with charged permeants have shown that the aqueous pores in the SC are charged, and that positively charged permeants traverse the skin more easily than negatively charged permeants (9,44). However, it is also known that the size of the permeant relative to that of the aqueous pore affects the penetration of the permeant into the skin (9,43). If the permeant is larger than the aqueous pore size, then electrostatic effects should be irrelevant, since the steric hindrance would prevent any access into the pore. However, when the permeant is physically small enough to access the skin aqueous pores, then the electrostatic interactions between the permeant and the pores, as well as the steric interactions between the permeant and the pore wall, will play a role in the transport of the permeant across the skin (9,12,43,45,47). In our experiments, all the micelies are negatively charged due to the presence of SDS,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)