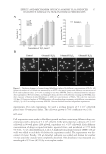
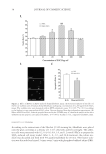
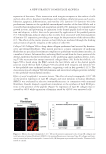
J. Cosmet. Sci., 64, 1–8 (January/February 2013) 1 Enzymatically generated hydrogen peroxide reduces the number of acne lesions in acne vulgaris NEELAM MUIZZUDDIN, STEVEN SCHNITTGER, WANDA MAHER, DANIEL H. MAES, and THOMAS MAMMONE, Estèe Lauder Companies, Melville, NY. Accepted for publication May 9, 2012. Synopsis A major component to the etiology of acne is the growth and invasion by Propionibacterium acnes. Hydrogen peroxide is an excellent antimicrobial agent but is unstable in most formulations. We have developed a hydrogen peroxide generation system using the enzyme glucose oxidase and glucose. This system is stable in a simple formulation and nonirritating. In a short-term clinical study (4 days), this formulation was effective in reducing the individual lesion size and total number of infl ammatory acne lesions. There was a 68% reduc- tion in acne-induced infl ammation and 61% reduction in acne size within 4 days of treatment. A long-term clinical study (6 weeks in use) displayed 56% reduction in total number of infl amed lesions and a 45% reduc- tion in noninfl amed lesions after 6 weeks. This suggests that topical enzymatically generated hydrogen peroxide may help alleviate acne. INTRODUCTION The pathogenesis of acne is complex, with strong evidence supporting the involvement of sebaceous hyperplasia, follicular hyperkeratinization, bacterial hypercolonization, as well as an immune reaction followed by infl ammation (1). The pathological process of acne starts with the production of excessive sebum. Sebum lipids are a complex mixture of squalene, cholesterol, wax esters, and triglycerides. The triglycerides can be metabolized by bacterial enzymes to glycerol and free fatty acids. Excessive sebum secretion and loosely bound corneocytes clog pores and create a moist, anaerobic environment where anaerobic microorganisms multiply and eventually pro- voke infl ammatory reactions. Excessive sebum production has been attributed to elevated androgen levels. Androgens have been shown to increase both the size and the output of sebum of the sebaceous gland (2). Acne begins to develop with the increase in androgens during the prepubertal period. Conversely, antiandrogens have been shown to reduce se- bum lipids and improve acne. Acne is also characterized by improper epidermal differentiation of the lower portion of the infundibulum of the sebaceous follicle, where keratinocytes lining the infundibulum are hyperproliferative compared with normal skin. This improper epidermal differentia- tion leads to a clogged pore, creating a microenvironment favorable for Propionibacterium
JOURNAL OF COSMETIC SCIENCE 2 acnes growth. The excessive lipids and improper differentiation of the keratinocytes in the follicle encourages bacterial growth and causes a weakening of the epidermal barrier in this follicle. This allows the bacteria and/or their metabolites to migrate into the skin and contribute to the infl ammation observed in the fi nal stages of the acne process (2–4). The fi nal phase of comedone formation is the infl amed lesion. The bacterial infi ltrate into the skin triggers infl ammatory mediator production and cellular infi ltrate. A variety of infl ammatory mediators have been described in the acne lesion. These include interleukin-1α (IL-1α), IL-1β, and substance P (5). In addition, there is a reported in- crease in lymphocytic infi ltrate and neutrophil infi ltrate in the follicular region (6), fur- ther contributing to the infl ammation associated with the acne lesion. This leads to further increased production of the proinfl ammatory cytokines, IL-1α and tumor necrosis factor-alpha by T cells and keratinocytes, leading to proliferation of both cell types (4). The primary pathogenic agent implicated in the development of infl ammatory and non- infl ammatory acne is P. acnes (7,8). P. acnes is included in a family of anaerobic, non-spore- forming gram-positive rods. The use of antibiotics to treat acne began in the 1960s however, in the last two decades several antibiotic-resistant strains have emerged (7–11). In addition to antibiotics, topical benzoyl peroxide (BP) (12,13) and salicylic acid have consistently been found to be effective in reducing acne lesions (14,15). Growing aware- ness of antibiotic-resistant Propionibacterium species has contributed toward increased use of topical and systemic differentiation agents like retinoid (16,17), which help reduce the hyperproliferation of keratinocytes and can inhibit the migration of leukocytes (1,6,9). Glucose oxidase catalyzes the oxidation of β-D-glucose to gluconic acid by utilizing mo- lecular oxygen as an electron acceptor with simultaneous production of hydrogen perox- ide (18). Such production of hydrogen peroxide from sources of glucose like honey has been shown to possess potent antibacterial properties (19) and has been used in wound healing (20,21). We have developed a hydrogen peroxide generating system using the enzyme glucose oxidase and glucose in an emulsion system that is stable and nonirritat- ing. This study was designed to evaluate the effect of this formulation system on reducing acne. METHODS AND MATERIALS IN VITRO CHALLENGE TEST An in vitro microbial challenge test was used to determine if the test material was effec- tive in eliminating microorganisms. Glucose oxidase (0.04%) and glucose substrate (0.36%), test material, were dissolved in tryptic soy broth (BD Difco, Sparks, MD) and challenged with fi ve pools of viable microorganisms known to contaminate cosmetics. Elimination of these microorganisms was followed over a 7-day period (22,23). Pool 1 Enterics contained Klebsiella pneumoniae (ATCC 1388), Escherichia coli (ATCC 8739), and Enterobacter gergoviae (ATCC 33028) Pool 2 Pseudomonas consisted of Burk- holderia cepacia (ATCC 25416), Pseudomonas stutzeri (ATCC 17588), Pseudomonas putida (ATCC 49128), and Pseudomonas aeruginosa (ATCC 9027) Pool 3 Staphylococci contained Staphylococcus aureus (ATCC 6538) and S. epidermidis (ATCC 49134) Pool 4 consisted of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)