ENZYMATICALLY GENERATED HYDROGEN PEROXIDE REDUCES ACNE LESIONS 3 yeast, Candida albicans (ATCC 10231) and Pool 5 consisted of mold Aspergillus niger (ATCC 16404). The test material was prepared in sterile tryptic soy broth (BD Difco) and 20 g of each was transferred aseptically into fi ve sterile containers. Each 20-g sample was inoculated with 0.1 ml of appropriate inoculums such that each bacteria and yeast pool contained 106 colonies, whereas the mold pool contained 105 colonies. The container was tightly closed and the inoculated sample was mixed thoroughly and then incubated at ambient temperature (20–25°C). The samples were observed for viable microbes after 24 h, 48 h, and 7 days. At each time point, 1.0 ml of each well-mixed sample was transferred by a sterile pipette to test tubes containing 9-ml diluent (Trypticase Azolectin Tween Broth Base BD Difco) creating a 1:10 dilution of which 1.1 ml was dispensed into one sterile petri dish and 1.0 ml into another. Melted agar medium (15–20 ml) kept at 45–50°C was added to each petri dish and rotated to disperse the product and agar thoroughly. Tryptic soy agar (BD Difco) was used for inoculum Pools 1, 2, and 3 and potato dextrose agar (BD Difco) for inoculum Pools 4 and 5. Once the agar was solidifi ed, the petri dishes were inverted and incubated at 32–35°C for 48 h (23). After 48 h incubation, the petri dishes were examined for recovery of any inoculated organ- ism. The number of colonies was counted on the petri dish representing the 1:10 dilution, and multiplied by 10, and then converted to its appropriate log value. The number of colo- nies counted on the petri dish representing the 1:100 dilution was multiplied by 100, and then converted to its appropriate log value. When there were no colonies present on the 1:10 or 1:100 dilution petri dishes, the count was represented by the log value of 0.0 (23). CLINICAL Part I: short-term effect. Material A: 10% BP formulation in an oil-in-water emulsion. Ma- terial B: glucose oxidase enzyme 0.08%, glucose substrate 0.64% in an oil-in-water emulsion. Material C: glucose oxidase enzyme 0.5%, glucose substrate 4.0% in an oil- in-water emulsion. Since the effect of hydrogen peroxide is pH dependent, the formulations were buffered at 7.0. There were no added ions such as copper and iron in these formulations. The effect of the above materials was evaluated in a 1-week assay described previously (21). Ten women volunteers between the ages of 18 and 50 were recruited from a local population. All subjects were healthy with no evidence of acute or chronic disease other than acne. Written informed consent was obtained from each volunteer before entrance into the study. The panelists were not on any antibiotic, antihistamines, retinoid, anti- infl ammatories or steroid therapy, and BP and/or salicylic acid treatment for at least 2 weeks before commencement of this study. The subjects were not under the care of a der- matologist and were not on any acne treatment for at least 1 month before the study started. Pregnant or lactating women were excluded. The panelists exhibited acne with at least two closed comedones on the upper back, minimum distances between closed comedones were approximately 4–6 cm. Two infl amed acne lesions were selected for each treatment and one for the untreated. Each lesion was marked, photographed, and graded. A skin surface microscope (Scopeman
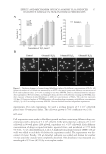
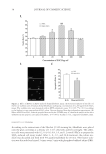
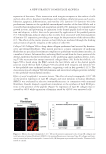
JOURNAL OF COSMETIC SCIENCE 4 Moritex, San Jose, CA) was used to visualize, size, and grade the lesion by two medical doctors at the testing lab. The lesions were treated and photographed every day for 5 days. Part II: long-term effect. Thirty-eight women between the ages of 18 and 50 were recruited for the study, following the same inclusion and exclusion criteria as described in Part I. The panel was divided into three groups as follows: (i) Group A (n = 15), 2.5% BP in an oil-in- water emulsion (ii) Group B (n = 14), 0.36% glucose oxidase substrate and 0.04% glucose oxidase enzyme in an oil-in-water emulsion and (iii) Group C (n = 9), untreated control. The formulations were buffered at pH 7.0. There were no added ions such as copper and iron in these formulations. The panelists exhibited Grade 2 acne (10–30 papules and comedones over about one- fourth of the face) to Grade 4 (about half the face had papules, comedones, and a few pustules some lesions were red and infl amed), or subtypes 1 (erythematotelangiectatic) and 2 (papulopustular) rosacea (National Rosacea Society). This was a 6-week in-use study where the subjects applied the test material on their full face, twice a day. They were examined at baseline (before treatment) and after 2 and 6 weeks of treatment with their assigned test materials. At each time point, lesions of the full face were counted by trained personnel at the contract testing laboratories. Both in- fl ammatory and noninfl ammatory lesions were counted. Since treatment was on full face every day, only the lower concentrations tested in the short-term assay were tested in the long-term assay, to avoid any irritation. RESULTS IN VITRO CHALLENGE TEST Microbiological challenge testing is a useful tool for determining the ability of a material to support the growth of spoilage organisms or pathogens. As observed in Table I, the glucose oxidase and glucose substrate mixture were very effective against Pools 1–3, but not against yeast and molds. CLINICAL Part I: short-term effect. Short-term lesion reduction study exhibited a marked reduction in lesion size on the site treated with the formulations containing glucose oxidase and glucose during course of the study (Figure 1A). As observed in the graph, there was a distinct reduc- tion in acne lesion size on the site treated with 0.5% glucose oxidase enzyme and 4% glu- cose (p = 0.041). The degree of infl ammation on acne sites is shown in Figure 1B. As observed in the graph, there was a marked reduction in acne lesion size on the site treated with the formulation containing 0.5% glucose oxidase enzyme and 4% glucose (p = 0.0467). The lower concentration of these actives was almost as effective as 10% BP, which did not exhibit statistically signifi cant improvement as compared to the untreated site. Area under the curves from Figures 1A and B are described in Figure 2. Area under the curve is a complete and comprehensive representation of the lesion over the course of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)