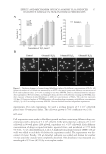
EFFECT AND MECHANISM OF EGCG AGAINST H2O2-INDUCED OXIDATIVE DAMAGE IN HUMAN DERMAL FIBROBLASTS 37 experiments. For each experiment, we used a seeding density of 5 × 104 cells/well plated onto 30-mm petri dishes. The cells were grown to 70% confl uency over 24 h. MTT ASSAY Cell suspensions were made in fi bro blast growth medium containing different drug con- centrations with a density of 5 × 104 cells/ml. Cells were plated at a density of 5 × 103 cells/well in 96-well plates (200 μl/well, equivalent to 1 × 104 cells/well). Each different concentration of drugs occupied six holes, repeated twice, and incubated at 37°C with 5% CO2. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (20 μl/ well) was added to each hole 4 h before the experiment ended. The supernatant was dis- carded 4 h later. Finally, 150 μl dimethyl sulfoxide was added and shaken for another 15 min until the crystals were completely dissolved. The absorbance was measured at a wavelength of 570 nm (A570) by a microplate reader. Figure 1. Oxidation damage to human dermal fi broblasts induced by different concentrations of H2O2. (A) Statistical analysis of cell death rate measured by an MTT colorimetric assay with different concentrations of H2O2 and treatment times. *p 0.05 according to one-way ANOVA. Data are obtained from fi ve indepen- dent experiments. (B) Cell viability and damaged cell nuclei detected by Hoechst staining and TUNEL assay after exposure of human dermal fi broblasts to different concentrations of H2O2. The scale bar represents 100 μm. (C) Statistical analysis of TUNEL-positive cells resulting from treatments with different concentrations of H2O2. *p 0.05 according to one-way ANOVA. Data are obtained from fi ve independent experiments.
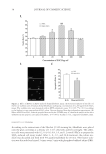
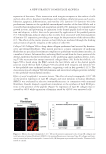
JOURNAL OF COSMETIC SCIENCE 38 HOECHST 33342 STAINING According to the instructions of the Hoechst 33342 staining kit, fi broblasts were plated onto the glass coverslips at a density of 1 × 104 cells/well, and left overnight. The adher- ent cells were pretreated with 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 mmol/l H2O2 to prepare the H2O2-induced cell injury model. After 3-, 6-, 12-, and 24-h treatment, the culture me- dium was discarded and fi xed with 4% paraformaldehyde for 20 min. The fi xative solu- tion was discarded and rinsed 3 times every 5 min with phosphate-buffered saline (PBS). Figure 2. Effect of EGCG on H2O2-induced dermal fi broblast injury. (A) Statistical analyses of the effect of EGCG on viability rates of human dermal fi broblasts undergoing concentration of 0–200 μg/ml with H2O2 injury. The viability rates were measured with an MTT colorimetric assay. *p 0.05 **p 0.01 according to the Stude nt’s t-test compared with H2O2 alone. Data are obtained from fi ve independent experiments. (B) Effect of EGCG on H2O2-induced apoptosis detected by the TUNEL assay. (C) Statistical analysis of the effect of EGCG on the apoptotic rate induced by H2O2. *p 0.05 by Student’s t-test, compared with H2O2 alone.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)