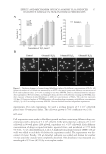
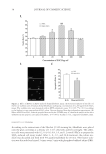
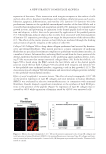
PREPARATION OF LIPOSOMES LOADED WITH AVO AND AR 17 sunscreen (AVO). The majority of AR in the lipo-AR-AVO, however, permeated through the membrane, which is essential for it to exhibit skin whitening effects after topical ap- plication. These results indicated that coencapsulated AR and AVO liposomes are a promising delivery system that could help retain the sunscreen on the surface of the skin for sun protection meanwhile aiding in the penetration of the whitening agent into the deeper layers of the skin for whitening effect. CONCLUSIONS Liposomal formulation of AR prepared by the reverse-phase evaporation method can ef- fectively enhance the entrapment of this highly hydrophilic compound in the hydrophilic core of the liposome. It may also increase the entrapment of other coencapsulated hydro- phobic compounds such as AVO into the lipid bilayer. Besides, coencapsulating AR and AVO has potential cosmetic applications in enhancing the stability and effi cacy of the compounds by retaining AVO at the surface meanwhile enhancing the penetration of AR into the deeper layers of the skin. This study provided a proof of concept that liposomal delivery system is a promising technical platform for coencapsulating cosmeceutical agents. REFERENCES (1) B. N. Barsoom, A. M. E. Abdelsa mad, and N. M. Adib, Indirect spectrophotometric determination of arbutin, whitening agent through oxidation by periodate and complexation with ferric chloride, Spectro- chim. Acta A, 64(4), 844–852 (2006). (2) M. Funayama, H. Arakawa, R. Yam amoto, T. Nishino, T. Shin, and S. Murao, Effects of alpha- and beta- arbutin on activity of tyrosinases from mushroom and mouse melanoma, Biosci. Biotech. Biochem., 59(1), 143–144 (1995). (3) K. Sugimoto, T. Nishimura, K. N omura, and T. Kuriki, Inhibitory effects of α-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model, Biol. Pharm. Bull., 27(4), 510–514 (2004). (4) S. Scalia and M. Mezzena, Co-load ing of a photostabilizer with the sunscreen agent, butyl methoxydibenzoylmethane in solid lipid microparticles, Drug Dev. Ind. Pharm., 35(2), 192–198 (2009). (5) C. Paris, V. Lhiaubet-Vallet, O. Jiménez, and M. Miranda, A blocked diketo form of avobenzone: Pho- tostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-fi lter, Photo- chem. Photobiol., 85(1), 178–184 (2009). (6) A. H. Wen, M. K. Choi, and D. D. K im, Formulation of liposome for topical delivery of arbutin, Arch. Pharm. Res., 29(12), 1187–1192 (2006). (7) C. Anselmi, M. Centini, C. Rossi, M. Ricci, A. Rastrelli, M. Andreassi, A. Buonocore, and C. La Rosa, New microencapsulated sunscreens: Technology and comparative evaluation, Int. J. Pharm., 242(1–2), 207–211 (2002). (8) V. Iannuccelli, N. Sala, R. Tursil li, G. Coppi, and S. Scalia, Infl uence of liposphere preparation on butyl- methoxydibenzoylmethane photostability, Eur. J. Pharm. Biopharm., 63(2), 140–145 (2006). (9) Y. Jing, C. J. Wiley, D. A. Godwin , and L. A. Felton, Infl uence of hydroxypropyl-b-cyclodextrin on transdermal penetration and photostability of avobenzone, Eur. J. Pharm. Biopharm., 69, 605–612 (2007). (10) G. M. N. Maghraby, A. C. Williams, and B. W. Barry, Can drug-bearing liposomes penetrate intact skin? J. Pharm. Pharmacol., 58(4), 415–429 (2006). (11) G. Barlett, Phosphorus assay in c olumn chromatography, J. Biol. Chem., 234, 466–468 (1959). (12) F. Szoka and D. Papahadjopoulos, Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation, Proc. Natl. Acad. Sci. USA, 75(9), 4194–4198 (1978).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)