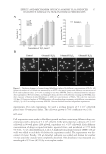
EFFECT AND MECHANISM OF EGCG AGAINST H2O2-INDUCED OXIDATIVE DAMAGE IN HUMAN DERMAL FIBROBLASTS 41 (Nanjing, China). Fibroblasts were plated onto 30-mm petri dishes with a density of 1 × 104 cells/dish, and left overnight. After the cells were completely adherent, they were incubated with H2O2 and H2O2 and EGCG for 6 h, respectively, and the cell protein was extracted. Finally, the contents of SOD, LPO product MDA and GSH-px were determined. DATA ANALYSIS SAS 6.0 was used for statistics analysis. Results were expressed as mean ± standard devia- tion. The analysis of variance (ANOVA) test was used for comparison among groups. The mean values between groups were compared using Student’s t-test. A p value 0.05 indicated a statistical signifi cance. RESULTS AND DISCUSSION H2O2-INDUCED HUMAN DERMAL FIBROBLASTS INJURY AND APOPTOSIS We fi rst used MTT assay to examine the survival rate of H2O2-induced human dermal fi broblasts and established the cell oxidative injury model. The H2O2 treatment time was 3, 6, 12, and 24 h with the concentration of 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2mmol/l. The results showed that the death rate was highly correlated with the concentration of the treatment, and was less correlated with the treatment time. At the same concentration, there is no signifi cant difference among the groups with different treatment time (Figure 1A). Based on the MTT assay results, we chose 6 h and 0.4–0.8mmol/l as the optimal induction conditions for the next series of experiments. Because the MTT assay could not show the kind of cell damage H2O2 treatment caused, we used Hoechst staining to deter- mine whether H2O2-induced fi broblast damage was associated with apoptosis. Hoechst 33342 is a specifi c fl uorescent dye that binds to the minor groove of DNA bases and can detect changes in the nuclear morphology of the apoptotic cells. Under the fl uorescence microscope, the control group showed uniformly stained larger nuclei and evenly dis- persed blue fl uorescence, whereas the H2O2-treated cells showed dense nuclei with brighter fl uorescence in blue and white than normal cells. In the nuclei of the apoptotic cells, dense granular fl uorescence was also observed. They exhibited a typical nuclear condensation and fragmentation, especially under H2O2 concentration of 0.8 mmol/l (Figure 1B, upper panel). We also used the TUNEL method to check the cell apoptosis under a fl uorescence microscope and semiquantitatively determined the rate of cell apop- tosis. The control group showed almost no green fl uorescence, whereas the H2O2-treated group showed positive green fl uorescent cells. The nuclei of the H2O2-treated group showed a typical form of apoptosis with nuclear condensation and fragmentation, and the number of apoptotic cells increased with the H2O2 concentration (Figure 1B, lower panel). In 100× high magnifi cation lens, the number of apoptotic cells randomly selected from fi ve discrete horizons in each group also increased with the H2O2 concentration (Figure 1C). It further confi rmed that H2O2-induced oxidative damage led to apoptosis. H2O2 is a reactive oxygen species with a high reactivity that can pro mote the generation of free radicals, causing membrane LPO, and can freely diffuse in the membrane, result- ing in cell necrosis and apoptosis. The result of the current study further consolidated this conclusion.
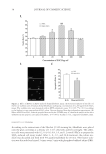
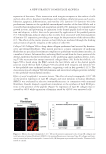
JOURNAL OF COSMETIC SCIENCE 42 EGCG PROTECTS CELLS AGAINST H2O2-INDUCED CELL INJURY AND APOPTOSIS We next studied whether the EGCG-treated human dermal fi broblasts had the ability to fi ght against the oxidative stress by using the H2O2-induced human dermal fi broblasts apoptotic model. We fi rst added EGCG with concentrations ranged from 10 to 200 μg/ml and tested the survival rate of the cells after H2O2 treatment with MTT assay. The results suggested that EGC effectively improved the survival rate of H2O2-treated cells. In particular, they showed signifi cant differences in 20, 50, and 100 μg/ml groups (p 0.05), and the 50 μg/ml group showed the most prominent effect. The statistics from three repeated experiments are shown in Figure 2A. The results from TUNEL assay experiments showed that by adding EGCG (50 μg/ml) the number of positive green fl uo- rescence cells is signifi cantly less than that of the cells treated with H2O2 only (Figure 2B). In 100× high magnifi cation lens, the number of apoptotic cells in the group treated with both H2O2 and EGCG was signifi cantly less than that in the group treated with H2O2 alone (p 0.05, Figure 1C). The above results indicated that EGCG had a protective effect against the H2O2-induced oxidative stress injury in human dermal fi broblasts. EFFECT OF EGCG ON DPPH RADICAL SCAVENGING We further studied whether EGCG has the ability to scavenge the free radicals using the DPPH radical spectrophotometric analysis. The effects of different concentrations of EGCG on free radical scavenging are shown in Figure 3. The results showed that with a concentration range of 1–200 μg/ml, EGCG had the ability to scavenge the free radicals and its effect increased as concentrations increased. With 200 μg/ml EGCG, its effect on radical scavenging reached up to 91.42%. Thus, EGCG has signifi cant effects on free radical scavenging. EFFECT OF EGCG ON SOD, GSH-PX ACTIVITY, AND MDA LEVEL OF HUMAN DERMAL FIBROBLASTS Lipid peroxide-mediated cell damage is one of the major causes of cell injury. MDA is the main product of LPO, and its level indirectly refl ects the degree of oxidative damage to cells. MDA level is also widely used as a biomarker for oxidative stress (16). In addition, antioxidant enzymes such as SOD and GSH-px are also considered to be effective in en- hancing the cellular antioxidant defense system (17). Therefore, we further explored the effects of EGCG on its protection against H2O2-induced cell oxidative stress and apopto- sis by measuring SOD, GSH-px activity, and MDA levels. The results showed that H2O2 dramatically reduced the SOD level in the human dermal fi broblasts compared to the control group. After adding EGCG, the SOD level could be signifi cantly recovered. Moreover, within the concentration range of 10–50 μg/ml, the effect of EGCG on recov- ery of SOD level was found to be concentration-dependent (Figure 4A). When the EGCG concentrations exceeded 50 μg/ml, the ability of EGCG on the recovery of SOD reduced and showed no signifi cant difference compared to the group treated with H2O2 alone (Figure 4A).This may result from the high concentration of EGCG-induced cell toxicity. EGCG also has effect on increasing the activity of GSH-px, which is another antioxidant enzyme. As shown in Figure 4B, H2O2 decreased the GSH-px activity of human dermal fi broblasts, and after adding EGCG, the GSH-px activity can be signifi cantly recovered. Similarly, the recovery of GSH-px activity showed a concentration-dependent effect when the EGCG concentration was in the range of 10–50 μg/ml. When the EGCG concentration
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)