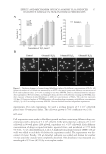
A NEW STRATEGY TO MODULATE ALOPECIA 49 5-α-REDUCTASE ACTIVITY The enzyme 5-α-reductase catalyzes the conversion of testosterone to DHT. The effects of biochanin A on 5-α-reductase activity were studied in intact cells expressing type 1 or type 2 isoforms of the enzyme and compared to that of epigallocatechin-3-gallate (EGCG) from green tea. EGCG is a known in vitro inhibitor of 5-α-reductase (23). In this assay, radio-labeled testosterone served as substrate. The activity of biochanin A on 5-α-reductase activity was shown by Hiipakka et al. (24). Briefl y, 5-α-reductase-expressing cells were plated at 50,000 per well in a 24-well plate in specifi c medium for 18 h at 37°C. The medium was then changed to 0.5 ml of serum- free medium and 5 μl of biochanin A (100 μM) or EGCG (100 μM) was added and kept for 1 h at 37°C before the addition of 14C-testosterone, at a fi nal concentration of 1.5 μM. Cells were then incubated for an additional 3 h and radioactive steroids were extracted with ethyl acetate. The amounts of labeled testosterone and DHT in extracts were next determined by thin layer chromatography, as a measure of 5-α-reductase activity (for more details, see Ref. 24). IMMUNOFLUORESCENT LABELING OF COLLAGEN III AND LAMININS The effect of acetyl tetrapeptide-3 on the expression of different ECM proteins (collagen III and laminins) was evaluated by selective immunofl uorescence in comparison with un- treated fi broblasts. For this experiment, 3 × 104 human fi broblasts (MRC5 from ATCC CCL) were incubated in Dulbecco’s modified Eagle’s medium (DMEM) (Eurobio Labora- tories, Courtaboeuf, France) containing 10% fetal calf serum (FCS) and supplemented with 1% penicillin/streptomycin. Cells were maintained in a humidifi ed incubator at 37°C with 5% CO2 atmosphere to reach confl uence. Cells were then incubated in the presence or absence of acetyl tetrapeptide-3 (10−7 M, equivalent to 0.05 ppm) for 3 days. These cells were rinsed with phosphate-buffered saline (PBS) and fi xed on slides using methanol (for 10 min at −20°C) followed by acetone fi xation (for 10 min, at 4°C). The slides were then dried at room temperature and rinsed with PBS at a pH of 7.6 for 10 min. The presence of collagen III and laminins in cells was detected by incubating the slides with specifi c antibodies diluted at 1/50e overnight at 4°C, that is, type III anti-collagen (rabbit, Rockland, Gilbertville, PA) and anti-laminin (rabbit, Sigma, St. Louis, MO), respectively. Detection of type III anti-collagen and anti-laminin antibodies was done using a goat anti-rabbit IgM + IgG rhodamine (TRITC) conjugate diluted at 1/100e (Southern Biotech, Birmingham, AL). The corresponding fl uorescent signal was moni- tored using confocal microscopy (Axioplan and Zeiss LSM510 Oberkochen, Germany), allowing for semiquantitative evaluation. IMMUNOHISTOLOGICAL LABELING OF COLLAGEN VII The effect of acetyl tetrapeptide-3 on the expression of collagen VII, a major constituent of anchoring fi brils found in the middle part of the follicular BMZ and around the hair papilla was evaluated using immunohistological techniques. As the junction around the anagen hair follicle and its adjacent connective tissue is similar in terms of composition
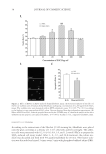
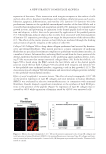
JOURNAL OF COSMETIC SCIENCE 50 and structure to the dermal–epidermal junction (DEJ) (25), the effect of acetyl tetrapeptide-3 on type VII collagen synthesis was evaluated on human skin explants. Four human skin explants were obtained from abdominal part of patients undergoing plastic surgery (Caucasian women, 35–45 years old) and maintained in culture. On day 1, a corticoid cream (Diprosone® with 0.05% betamethasone) was applied at the surface of the skin explants to induce skin atrophy as seen in aging (26). Acetyl tetrapeptide-3 was then added to the culture media for 2 days at 10−5 M concentration (equivalent to 5 ppm). On day 3, the skin explants were prepared for specifi c collagen VII immunohistological labeling using Avidin-Biotin Complex ABC Peroxidase Kit (Vector Laboratories Burlingame, CA, USA) and revealed by AEC substrate (brown color). Visual scoring of collagen VII expression (n = 4) was performed, using a scale ranging from 0 (negative) to 4 (maximum) defi ned as follows: 0, absence of the protein 1, slight expression 2, moderate expression 3, normal expression and 4, overexpression. MODULATION OF IL-8 PRODUCTION Low-grade chronic infl ammation is increasingly seen as a contributing factor in male pattern alopecia (27). Under stress conditions, keratinocytes in the vicinity of the hair follicle may respond by releasing IL-1, a proinfl ammatory cytokine that commands the production of additional infl ammatory agents such as IL-8 acting as chemoattractants for infl ammatory cells. IL-1-induced IL-8 production in keratinocytes was used as a model to document the anti-infl ammatory activity of red clover extract alone and in combination with acetyl tetrapeptide-3. Dexamethasone (DMS), a glucocorticoid with potent anti- infl ammatory properties, served as a positive control for anti-infl ammatory action. Monolayers of cells derived from normal human dermal fi broblasts (NHDFs) (Life tech- nologies, Saint Aubin, France) were cultured to confl uence for 24 h in DMEM (Eurobio Laboratories) containing 10% FCS and supplemented with 200 mM L-glutamine and 1% penicillin/streptomycin in a humidifi ed incubator at 37°C with 5% CO2 atmosphere. Cells were then challenged by adding IL-1α (0.0075 ng/ml from Eurobio Laboratories) to the culture media (without FCS) in the presence or absence of the test products (red clover extract alone or red clover extract + acetyl tetrapeptide-3) (0.5–1%) or DMS (1 μM) for 24 h. At the end of this period, IL-1α-induced IL-8 production was measured using a highly sensitive and specifi c enzyme immunoassay kit (human CXCL8/IL-8 DuoSet R&D system Minneapolis, MN). CLINICAL EFFICACY Study population. Thirty healthy volunteers with active mild to moderate hair loss enrolled in the study. Patients were clinically evaluated and individual case histories were recorded to rule out possible pathologies such as iron defi ciency anemia, thyroid-related condi- tions, or others that may infl uence hair growth. Patients were asked to use only “basic” shampoo and to avoid hair care treatment lotion according to the protocol. Hair count evaluation was done at the preselection step. As an inclusion criterion, less than 70% of all hair had to be in the anagen phase.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)