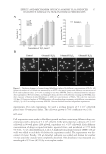
PREPARATION OF LIPOSOMES LOADED WITH AVO AND AR 15 and 59.7%, respectively. It was diffi cult to obtain high EE for AR, a highly hydrophilic com- pound, by simply using the thin fi lm hydration method for liposome preparation. It is well known that liposomes prepared by the thin fi lm hydration method generate multilamellar vesicles (MLVs). In MLVs, most of the lipids participate in forming the internal lamellae with tight lipid bilayers, which limits their internal aqueous space. Although applying sonication and high-pressure extrusion would yield single unilamellar vesicles with larger encapsulation volume, these techniques, nonetheless, will result in low aqueous space to lipid ratio and thereby low EE for hydrophilic drugs in liposomes (12). In contrast, reverse-phase evaporation method has unique advantages for encapsulating water-soluble materials such as proteins, nucleic acids, and other biochemical reagents (12). With reverse-phase evaporation method, the organic solvent is simply removed from the inverted micelles resulting in vesicles with larger aqueous space to lipid ratio and consequently higher EE. The EE of AR and AVO increased to 7.2% and 76.4%, respectively, in liposomes made by the reverse-phase evaporation method. Entrapment was depended on EPC whereby the entrap- ment of AR increased by up to 2.2-folds when the concentration of EPC increased to 20 μmol. These results indicated that the reverse-phase evaporation method is more effi cient for prepar- ing liposomes co-encapsulating AR and AVO with high EE. The particle size of the liposomes prepared by either the thin fi lm hydration or the reverse-phase evaporation methods is given in Table I. Data show that the particle size of liposomes was in the range of 150–400 nm. The stability of the coencapsulated AR and AVO liposomes was evaluated by monitoring the change in particle size and polydispersity index (PI) of liposomes stored for up to 30 days at 4°C and controlled room temperature. The average sizes of the liposomes in- creased within 1 day and remained relatively constant throughout the 30 days of storage at either 4°C or 25°C (Table II). Upon visual inspection, no signs of drug precipitation were observed. Furthermore, in preliminary studies, it was seen that expulsion of drugs from the liposomes would result in signifi cant change in the size of the particles. There- fore, results shown in Table II indirectly indicate that the liposomes were stable through- out the storage period. Nonetheless, additional studies are planned to confi rm the entrapment of AR and AVO within the liposome. IN VITRO PERMEATION STUDY In this study, the permeation of individual and coencapsulated AR and AVO lipo- somes made by the reverse-phase evaporation method was evaluated by measuring the Table II Effect of Storage Temperature and Time on the Particle Size Distribution of Lipo-AR-AVO Storage condition duration (day) 4°C 25°C Size (nm) PI Size (nm) PI 1 228.6 ± 17.1 0.37 ± 0.09 231.9 ± 25.4 0.40 ± 0.12 3 226.2 ± 8.6 0.41 ± 0.09 233.8 ± 13.2 0.40 ± 0.08 10 229.6 ± 16.0 0.44 ± 0.11 225.0 ± 12.3 0.36 ± 0.09 30 229.2 ± 10.7 0.55 ± 0.10 212.8 ± 13.5 0.40 ± 0.13 Data represent mean ± standard deviation (N = 3).
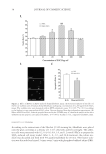
JOURNAL OF COSMETIC SCIENCE 16 concentration of the compounds that have permeated through the cellulose acetate mem- brane after 6 h of treatment. The distribution of individual AR and AVO in the donor and receptor compartments and their accumulation on the membrane is shown in Figure 2A. Only approximately 15% of AR was left in the donor side. Nonetheless, the percent- age of AR retained by the membrane was similar to the percentage of AR that permeated into the receptor compartment. A signifi cantly larger percentage of AR permeated into the receptor compartment when AR was formulated into lipo-AR-AVO (Figure 2B). With regard to AVO, insignifi cant amount of the compound remained in the donor side, and no AVO was detected in the receptor side. The majority of AVO, either in lipo-AVO or in lipo-AR-AVO formulations, was retained by the membrane. The fact that lipo-AR- AVO resulted in the accumulation of AVO and approximately 30–40% of AR on the membrane while allowing the remainder of AR to permeate through the membrane dem- onstrated the potential benefi ts of liposomes in the topical delivery of AVO and AR. The accumulation of the compounds on the membrane mimics their accumulation on stratum corneum. In topical applications, it is ideal to prevent the permeation of chemical sun- screens through the stratum corneum into the dermis layer for subsequent systemic cir- culation. The retention of some AR on the membrane could also help stabilize the Figure 2. Results from the in vitro permeability study showing (A) the distribution of AR and AVO in the donor side, receptor side, and the membrane when administered individually as lipo-AR and lipo-AVO (N = 3), and (B) the distribution of AR and AVO in the donor side, receptor side, and the membrane when administered coencapsulated within a liposomal formulation (lipo-AR-AVO, N = 3).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)