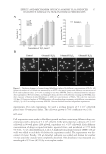
PARABENS IN COMMERCIAL COSMETICS 71 Table IV Results of the Contents of Preservatives in 105 for Children’s Cosmetics Category Methylparaben Ethylparaben Propylparaben Butylparaben Mean Minimum Maximum Mean Minimum Maximum Mean Minimum Maximum Mean Minimum Maximum Leave-on products 0.08 0.008 0.35 0.06 0.005 0.22 0.05 0.002 0.38 0.03 0.002 0.08 Rinse-off products 0.04 0.001 0.13 ND ND ND 0.03 0.001 0.11 ND ND ND ND: Not detected.
JOURNAL OF COSMETIC SCIENCE 72 CONCLUSIONS In this survey, contents of parabens detected in cosmetics for children in Beijing, China, were under the restricted levels. The detection rate of methylparaben in the 105 products for children was the highest, which is similar to the results of Guangdong Province and Shenzhen City. It is worth noting that the dosages of parabens found in leave-on product samples were higher than those in rinse-off product samples. Since the levels of paraben preservatives in children’s cosmetics were at the same level as those in adults’ cosmetics while infants and children’s skin is more permeable than that of adults, further research should be performed to prove whether the level of parabens in children’s cosmetics is harmful. REFERENCES (1) S. Q. Feng, View on the trend of preservatives in cosmetics from frequency of use (to be continued), Detergent & Cosmetics, 29, 30–33 (2006). (2) S. Q. Feng, View on the trend of preservatives in cosmetics from frequency of use, Detergent & Cosmetics, 29, 32–35 (2006). (3) Z. F. Deng, Y. Liu, D. A. Du, Z. M. Zeng, H. G. Zhong, and X. L. Jin, Investigation and analysis on preservatives of cosmetics in Guangdong Province, J. Environ. Health, 18, 89–91 (2001). (4) J. J. Dai, F. Liu, W. Liang, C. M. Situ, Y. Zhang, and F. Qiu, Analysis of the preservatives in cosmetics sold in Shenzhen, Chin. J. Health Lab. Technol., 14, 477–478 (2004). (5) No authors listed, Final amended report on the safety assessment of methylparaben, ethylparaben, pro- pylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products, Int. J. Toxicol.,27, 1–82 (2008). (6) T. T. Vo and E. B. Jeung, An evaluation of estrogenic activity of parabens using uterine calbindin-d9k gene in an immature rat model, Toxicol. Sci., 112, 68–77 (2009). (7) H. Yang, T. T. Nguyen, B. S. An, K. C. Choi, and E. B. Jeung, Synergistic effects of parabens on the induction of calbindin-D(9k) gene expression act via a progesterone receptor-mediated pathway in GH3 cells, Hum. Exp. Toxicol., 31, 134–144 (2012). (8) T. G. Zhao, Hygienic Standard for Cosmetics Health (Military Medical Press, Beijing, 2007), pp.75–241. (9) S. Pedersen, F. Marra, S. Nicoli, and P. Santi, In vitro skin permeation and retention of parabens from cosmetic formulations, Int. J. Cosmet. Sci., 29, 361–367 (2007). (10) P. Pigatto, A. Martelli, C. Marsili, and A. Fiocchi, Contact dermatitis in children, Ital. J. Pediatr., 36, 2 (2010).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)