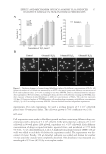
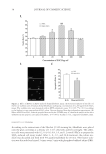
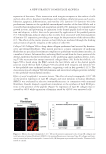
EFFECT AND MECHANISM OF EGCG AGAINST H2O2-INDUCED OXIDATIVE DAMAGE IN HUMAN DERMAL FIBROBLASTS 43 exceeded 50 μg/ml, the ability of EGCG on the recovery of GSH-px activity also de- creased, showing no signifi cant difference compared to the group treated with H2O2 alone (Figure 4B). This result further supported the cell toxicity caused by the high con- centration of EGCG. Similar conclusions were also obtained from the experiments of MDA level measure- ments. As shown in Figure 4C, H2O2 increased the MDA level of human dermal fi bro- blasts and after adding EGCG, the increased MDA level was signifi cantly reduced. However, this effect disappeared under high concentrations of EGCG. In summary, our results showed that EGCG can increase endogenous antioxidant enzymes activity (SOD and GSH-px) and can enhance the OFR scavenging capacity of human body’s own antioxidant defense system while reducing the MDA level. CONCLUSIONS In this study, for the fi rst time we have established the H2O2-induced human dermal fi broblasts as an oxidative injury model. By using different methods such as MTT assay, Hoechst staining, and the in situ TUNEL assay, we confi rmed that H2O2 could inhibit the viability of human dermal fi broblasts and induced the apoptosis at a certain concen- tration. EGCG showed a good protective antioxidative effect and signifi cantly inhibited the oxidation-induced apoptosis in the TUNEL experiments. We also tried to detect the apoptosis by using fl ow cytometer. However, the result is not as convincing as the TUNEL experiments. This study also confi rmed that EGCG increases SOD and GSH-px activity in human dermal fi broblasts and lowers the MDA level, signifi cantly inhibits the LPO in the human dermal fi broblasts, and shows a clear protective effect against H2O2 damage. Therefore, this method provides a theoretical basis for the application of EGCG in cos- metics and is a simple, fast way to screen the cosmetics with antioxidant effect. This is also worthy of further research and be a useful application in terms of screening cosmetics raw materials. ACKNOWLEDGMENT The authors thank Dr. Yan-Ai Mei, professor at School of Life Sciences, Fudan University, for valuable comments on the manuscript. REFERENCES (1) J. H. Chung, S. H. Youn, O. S. Kwon, H. C. Eun, K. H. Kim, K. C. Park, K. H. Cho, and J. I. Youn, Enhanced proliferation and collagen synthesis of human dermal fi broblasts in chronically photodam- aged skin, Photodermatol. Photoimmunol. Photomed., 12, 84–89 (1996). (2) H. G. Vogel, Age-dependent changes in skin biomechanics, measurements in vitro and in vivo. Z. Gerontol., 27, 182–185 (1994). (3) Y. Nishimori, C. Edwards, A. Pearse, K. Matsumoto, M. Kawai, and R. Marks, Degenerative alterations of dermal collagen fi ber bundles in photodamaged human skin and UV-irradiated hairless mouse skin: Possible effect on decreasing skin mechanical properties and appearance of wrinkles, J. Invest. Dermatol., 117, 1458–1463 (2001).
JOURNAL OF COSMETIC SCIENCE 44 (4) C. T. Ho, Q. Chen, H. Shi, K. Q. Zhang, and R. T. Rosen, Antioxidative effect of polyphenol extract prepared from various Chinese teas, Prev. Med., 21, 520–525 (1992). (5) S. K. Katiyar, R. Agarwal, M. T. Zaim, and H. Mukhtar, Protection against N-nitrosodiethylamine and benzopyrene-induced forestomach and lung tumorigenesis in A/J mice by green tea, Carcinogenesis, 14, 849–855 (1993). (6) C. S. Yang, J. D. Lambert, and S. Sang, Antioxidative and anti-carcinogenic activities of tea polyphe- nols, Arch. Toxicol., 83, 11–21 (2009). (7) J. M. Landau, Z. Y. Wanf, G. Y. Yang, W. Ding, and C. S. Yang, Inhibition of spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice by black and green tea, Carcinogenesis, 19, 501–507 (1998). (8) T. C. Hour, Y. C. Liang, I. S. Chu, and J. K. Lin, Inhibition of eleven mutagens by various tea extracts, (-)-epigallocatechin-3-gallate, gallic acid and caffeine, Food Chem. Toxicol., 37, 569–579 (1999). (9) J. Y. Bae, J. S. Choi, Y. J. Choi, S. Y. Shin, S. W. Kang, S. J. Han, and Y. H. Kang, (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fi broblasts: Involvement of mitogen-activated protein kinase, Food Chem. Toxicol., 46, 1298–1307 (2008). (10) J. Kima, J. S. Hwangb, Y. K. Chob, Y. Hanb, Y. J. Jeona, and K. H. Yanga, Protective effects of (-)-epigallocatechin-3-gallate on UVA and UVB-induced skin damage, Skin Pharmacol. Appl. Skin Physiol., 14, 11–19 (2001). (11) A. Dooley, S. W. Xu, N. Aden, T. Tranah, N. Desai, C. P. Denton, D. J. Abraham, and R. Bruckdorfer, Modulation of collagen type I, fi bronectin and dermal fi broblast function and activity, in systemic scle- rosis by the antioxidant epigallocatechin-3-gallate, Rheumatology, 49, 2024–2036 (2010). (12) P. Brenneisen, J. Wenk, M. Wlaschek, R. Blaudschun, and K. S. Kochanek, A newly adapted pulsed- fi eld gel electrophoresis technique allows to detect distinct types of DNA damage at low frequencies in human dermal fi broblasts upon exposure to non-toxic H2O2 concentrations, Free Rad. Res., 31, 405–418 (1999). (13) Y. Zheng, H. J. Song, C. H. Kim, H. S. Kim, E. G. Kim, A. Sachinidis, and H. Y. Ahn, Inhibitory effect of epigallocatechin 3-O-gallate on vascular smooth muscle cell hypertrophy induced by angiotensin II, J. Cardiovasc. Pharmacol., 43, 200–208 (2004). (14) C. S. Yang, X. Wang, G. Lu, and S. C. Picinich, Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance, Nat. Rev. Cancer 9, 429–439 (2009). (15) M. Kanadzu, Y. Lu, and K. Morimoto, Dual function of (K)-epigallocatechin gallate (EGCG) in healthy human lymphocytes, Cancer Lett., 241, 250–255 (2006). (16) M. Cini, R. G. Fariello, A. Bianchetti, and A. Moretti, Studies on lipid peroxidation in the rat brain, Neurochem. Res., 19, 283–288 (1994). (17) T. Luo and Z. Xia, A small dose of hydrogen peroxide enhances tumor necrosis factor-alpha toxicity in inducing human vascular endothelial cell apoptosis: Reversal with propofol. Anesth. Analg., 103(1), 110–116 (2006).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)