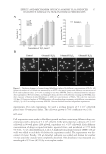
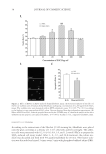
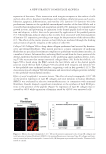
J. Cosmet. Sci., 64, 35–44 (January/February 2013) 35 Effect and mechanism of epigallocatechin-3-gallate (EGCG). against the hydrogen peroxide-induced oxidative damage in human dermal fi broblasts BING FENG, YUN FANG, and SHAO-MIN WEI, School of Chemical and Material Engineering, Jiangnan University, Wuxi, Jiangsu 214122 (B.F., Y.F., S.-M.W.) and R&D Center, Shanghai Jahwa United Co. Ltd., Shanghai 201702 (B.F., S.-M.W.), People’s Republic of China. Accepted for publication June 5, 2012. Synopsis This study was conducted to investigate the protective effects of epigallocatechin-3-gallate (EGCG) on hydrogen peroxide (H2O2)-induced oxidative stress injury in human dermal fi broblasts. 3-(4,5-Dimethylthi- azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay and the use of Hoechst staining and terminal deoxynucleotidyl transferase dUTP nick end labeling for apoptosis detection indicated that the administration of H2O2 to human dermal fi broblasts caused cell damage and apoptosis. The incubation of human dermal fi broblasts with EGCG markedly inhibited the human dermal fi broblast injury induced by H2O2. The assay for 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity indicated that EGCG had a direct, concentration-dependent antioxidant activity. Treatment of human dermal fi broblasts with EGCG signifi cantly reversed the H2O2-induced decrease of superoxide dismutase (SOD) and glutathione peroxidase (GSH-px), and the inhibition of malondialdehyde (MDA) levels. These results showed that EGCG possessed antioxidant activity and was effective against H2O2-induced human dermal fi broblast injury by enhancing the activity of SOD and GSH-px, and by decreasing the MDA level. Our results suggested that EGCG should have the potential to be used further in cosmetics and in the prevention of aging-related skin injuries. INTRODUCTION Human dermal fi broblasts are the predominant cell type in the dermis and play an im- portant role in the aging process. Apoptosis of human dermal fi broblasts can decrease cell number and cell activity, leading to reduced synthesis of collagen and other extracellular matrix and fi nally to change in morphology and function of dermal layer (1–3). The clinical symptoms include many skin aging changes such as dry rough skin, loose skin, reduced elasticity, and increased wrinkles. Therefore, the study of human dermal fi broblasts apopto- sis is crucial for the research and development of personal antiaging protective products. Address all correspondence to Shao-Min Wei at weishaomin@jahwa.com.cn.
JOURNAL OF COSMETIC SCIENCE 36 Green tea contains polyphenolic compounds, mainly catechin, which is further composed of several monomers including epicatechin, epicatechin gallate, epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG). Among all the monomers, EGCG has the highest proportion, accounting for about 50% of catechins (4,5). It has a strong antioxidant activ- ity and can effectively remove the active free radicals within human body, inhibit lipid peroxidation (LPO), and reduce oxygen free radical (OFR) and LPO-induced damage to cells, DNA, or other biological macromolecules (6–8). Through its strong antioxidant effects, EGCG can also reduce the intracellular reactive oxygen species activity, block the ultraviolet (UV) radiation, inhibit the matrix metalloproteinase activation and collagen damage on human dermal fi broblasts, and increase the extracellular matrix (9–11). Exogenous sources for the production of cellular hydrogen peroxide (H2O2), especially in the skin, are UVA and UVB irradiation (12). In the study of skin photoaging, the most common skin lesions are caused by chronic UV irradiation, which involves the demise, aging, and apoptosis of human dermal fi broblasts. Therefore, the establishment of an in vitro model of H2O2-induced human dermal fi broblasts apoptosis is crucial for future studies on the oxidative damage of human skin, screening of antioxidants, and its application in the cosmetics. Although many studies have reported that EGCG has strong antioxidant activity (13,14), little is known about its role in the model of H2O2-induced human der- mal fi broblast apoptosis (15). In this study, for the fi rst time, we address the effect of EGCG on oxidative damage and apoptosis induced by H2O2 in human dermal fi broblasts and explore the mechanism of its protective role, which provides a theoretical basis for the application of EGCG in cosmetics. In this study, we used H2O2-induced oxidative damage in human dermal fi broblasts as a model to study the effects of EGCG on H2O2-induced oxidative damage and apoptosis and examined its underlying mechanism. MATERIALS AND METHODS MATERIALS Dulbecco’s modifi ed Eagle’s medium (DMEM) and fetal calf serum (FCS) were purchased from Gibco Life Technologies (Grand Island, NY). H2O2 (3%), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Propidium Iodide (PI), and EGCG (purity 98%) were purchased from Sigma-Aldrich (St. Louis, MO). Terminal deoxynucleo tidyl tran sferase dUTP nick end labeling (TUNEL) apoptosis detection kit was purchased from Roche (Basel, Swit- zerland). Superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione per- oxidase (GSH-px) detection kit were purchased from Jiancheng Biological Engineering Academy (Nanjing, China). CELL CULTURE Primary cultures of skin fi broblasts from a healthy boy’s foreskin left over from surgery were grown on plastic fl as ks under standard conditions: DMEM supplemente d with 10% CS at 37°C in a humidifi ed atmosphere of 5% CO2. According to the different pur- poses of experiments, cells were quantitatively planted onto the cell culture bottles or 30-mm petri dishes for future experiments. Third- to sixth-generation cells were used for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)