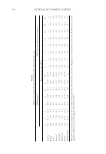
Table III AUC0–52Values Obtained Pretreating Skin Sites With Formulation D, SLN-IN,and SLN-OUT Containing SG (0.5% w/w) or With Formulation G Containing DG (0.5% w/w) and Applying UVB Radiations After 1 h (=1), 3 h (=3), or 6 h (=6) From Their Removal Subjects AUC0–52 t = 1 t 3 t = 6 Control D G SLN-IN SNL-OUT D G SLN-IN SNL-OUT D G SLN-IN SNL-OUT A 690.1 747.3 1218.6 928.6 1019.2 1128.3 918.2 1116.3 1278.2 1266.5 796.2 1201.2 1418.2 B 715.3 791.2 1200.4 900.3 1115.6 1215.6 924.3 1022.4 1266.3 1288.4 774.3 1210.2 1575.6 C 628.6 801.3 1229.3 1017.1 998.2 1117.3 896.2 1128.3 1215.9 1321.3 726.5 1204.3 1449.8 D 657.8 816.1 1244.8 949.4 1011.2 1198.3 865.4 1129.4 1298.8 1304.5 748.6 1198.6 1320.6 E 731.4 788.4 1197.3 1000.3 1103.9 1088.3 928.3 1138.2 1299.5 1316.6 702.1 1192.1 1146.8 F 726.9 809.3 1208.1 921.3 1088.2 1156.2 919.3 1100.1 1264.6 1324.9 721.2 1194.3 1294.3 G 697.1 826.4 1224.3 916.2 963.4 1228.4 926.2 1099.6 1228.1 1288.6 716.4 1218.4 1421.2 H 701.3 747.3 1281.6 928.4 943.1 1103.6 888.2 1098.4 1300.4 1216.5 698.6 1221.6 1318.3 I 721.4 768.1 1291.5 896.5 995.9 1163.2 826.3 1122.2 1318.2 1284.4 688.5 1244.3 1221.1 L 654.1 724.4 1196.3 886.4 912.2 1212.8 849.5 1211 1294.6 1296.3 788.4 1198.6 1393.0 Mean 696.66 788.38 1232.88 939.79 1026.52 1155.47 899.16 1106.1 1274.44 1290.19 730.27 1209.44 1355.9 TOPICAL DELIVERY OF ANTI-INFLAMMATORY COMPOUNDS 351
JOURNAL OF COSMETIC SCIENCE 352 a rapid increasing of SG and DG percutaneous absorption and consequently an anti- infl ammatory effect that occurs within the fi rst 3 h. Instead the above mentioned fusion of SLN lipid matrix with stratum corneum lipids, could lead to the formation of a reser- voir able to realize a SG sustained release toward deeper skin layers and consequently a prolonged anti-infl ammatory activity. In conclusion, the use of both penetration enhancers and SLN resulted to be valid tools to optimize the topical delivery of DG and SG. In particular, the use of soy lecithin guaranteed an increase in the percutaneous absorption of the two actives and a rapid anti- infl ammatory effect in in vivo experiments. Otherwise the results of the present study revealed an interesting delayed and sustained activity of SG-loaded SLN. Further in vitro and in vivo studies are in progress with the aim to maximize the results obtained with these two different strategies. REFERENCES (1) M. N. Asl and H. Hosseinzadeh, Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds, Phytother. Res., 22, 709–724 (2008). (2) G. L. Capella and A. F. Finzi, Complementary therapy for psoriasis, Dermatol. Ther., 16, 164–174 (2003). (3) S. Yano, Experiment on the essentials of corticoid activity of glycyrrhizin,. J. Jpn. Assoc. Endoc., 34, 745–751 (1958). (4) M. Kerube, Study on mechanism of anti-infl ammatory action of glycyrrhetinic acid, J. Keio. Med. Soc., 47, 331–344 (1970). (5) J. Hadgraft, “Formulation of Anti-Infl ammatory Agents,” in Nonsteroidal Anti-Infl ammatory Drugs, Phar- macology and Skin, C. Hensby and N. J. Lowe. Eds. (Karger, Basel, 1989), Vol. 2, pp. 21–43. (6) A. C. Williams and B. W. Barry, Penetration enhancers, Adv. Drug Deliv. Rev., 56, 603–618 (2004). (7) A. G. Doukas and N. Kollias, Transdermal drug delivery with a pressure wave, Adv. Drug Deliv. Rev., 56, 559–579 (2004). (8) C. Puglia, R. Filosa, A. Peduto, P. de Caprariis, L. Rizza, F. Bonina, and P. Blasi, Evaluation of alternative strategies to optimize ketorolac transdermal delivery, AAPS Pharm. Sci. Tech., 7, 64 (2006). (9) J. Hadgraft, Skin: The fi nal frontier, Int. J. Pharm., 224, 1–18 (2001). (10) R. H. Muller, M. Radtke, and S. A. Wissing, Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations, Adv. Drug Deliv. Rev., 54, S131–S155 (2002). (11) C. Puglia and F. Bonina, Lipid nanoparticles as novel delivery systems for cosmetics and dermal phar- maceuticals, Expert Opin. Drug Deliv., 9, 429–441 (2012). (12) M. Ricci, C. Puglia, F. Bonina, C. Di Giovanni, S. Giovagnoli, and C. Rossi, Evaluation of indomethacin percutaneous absorption from nanostructured lipid carriers (NLC): In vitro and in vivo studies, J. Pharm. Sci., 94, 1149–1159 (2005). (13) C. Puglia, P. Blasi, L. Rizza, A. Schoubben, M. Ricci, F. Bonina, and C. Rossi, Lipid nanoparticles for prolonged topical delivery: An in vitro and in vivo investigation, Int. J. Pharm., 357, 295–304 (2008). (14) K. Mitri, R. Shegokar, S. Gohla, C. Anselmi, and R. H. Müller, Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability, and performance, Int. J. Pharm., 414, 267–275 (2011). (15) C. Puglia, G. Frasca, T. Musumeci, L. Rizza, G. Puglisi, F. Bonina, and S. Chiechio, Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice, Eur. J. Pharm. Biopharm., 81, 288–293 (2012). (16) A. M. Kligman and E. Christophers, Preparation of isolated sheets of human stratum corneum, Arch. Dermatol., 88, 702–705 (1963). (17) J. Swarbrick, G. Lee, and J. Brom, Drug permeation through human skin. I: Effects of storage condi- tions of skin, J. Invest. Dermatol., 78, 63–66 (1982). (18) R. L. Bronaugh, R. F. Stewart, and M. Simon, Methods for in vitro percutaneous absorption studies VII: Use of excised human skin, J. Pharm. Sci., 75, 1094–1097 (1986). (19) E. Touitou and B. Fabin, Altered skin permeation of a highly lipophilic molecule: Tetrahydrocannabi- nol, Int. J. Pharm., 43, 17–22 (1988).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)