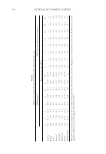
HPLC DETERMINATION OF HINOKITIOL 387 Figure 5. Typical chromatogram of a skin lotion sample (500 μl/100 ml, 200-fold diluted) after derivatiza- tion with NBD-F. The sample was reacted with NBD-F for 10 min in borate buffer, pH 9.0, at 60°C. Reten- tion time of NBD-hinokitiol: 7.2 min (arrowed peak). 4-hydroxybenzoate under derivatization (60°C, pH 9.0, 10 min), further studies are needed to solute the point. APPLICATION TO SKIN LOTION Figure 5 shows a typical chromatogram obtained from a skin lotion sample. The peak of NBD-hinokitiol was detected at 7.2 min, and the concentration of hinokitiol in the cos- metic was determined as follows. The described method was used to determine hinokitiol in the skin lotion and in samples spiked with standards. As shown in Table III, the concentration of hinokitiol in the skin
JOURNAL OF COSMETIC SCIENCE 388 lotion was found to be 194 ± 14 μg/ml (range: 180–212 μg/ml). Recovery of spiked hi- nokitiol from the lotion was 91.8 ± 3.7% (range: 84.5–98.0%). CONCLUSION We have developed a simple HPLC–UV method for the determination of hinokitiol after precolumn derivatization with NBD-F. Although the sensitivity of our method can be classed as only moderate compared with other reported methods, it is superior to that of our previous method. The newly developed HPLC method is simple, convenient, and suffi ciently sensitive for routine assay of hinokitiol, for example in quality control for personal care products. REFERENCES (1) J. Nault, A capillary gas chromatographic method for thujaplicins in western red cedar extractives, Wood Sci. Technol., 21, 311–316 (1987). (2) Y. Inamori, S. Shinohara, H. Tsujibo, T. Okabe, Y. Morita, Y. Sakagami, Y. Kumeda, and N. Ishida, Antimicrobial activity and metalloprotease inhibition of hinokitiol-related compounds, the constitu- ents of Thujopsis dolabrata S. and Z. hondai MAK, Biol. Pharm. Bull., 22, 990–993 (1999). (3) Y. Inamori, Y. Sakagami, Y. Morita, M. Shibata, M. Sugiura, Y. Kumeda, T. Okabe, H. Tsujibo, and N. Ishida, Antifungal activity of hinokitiol-related compounds on wood-rotting fungi and their insec- ticidal activities, Biol. Pharm. Bull., 23, 995–997 (2000). (4) E. L. Johnson and A. J. Cserjesi, Gas-liquid chromatography of some tropolone-TMS ethers, J. Chro- matogr., 107, 388 (1975). (5) F. Hanafusa, K. Nakamura, S. Togano, and T. Ohta, Determination of hinokitiol in cosmetic products by HPLC, Bunseki Kagaku, 38, 124–128 (1989). (6) M. Endo, T. Mizutani, M. Matsumura, M. Moriyasu, M. Ichimaru, A. Kato, and Y. Hashimoto, High- performance liquid chromatographic determination of hinokitiol in cosmetics by the formation of difl u- oroborane compounds, J. Chromatogr., 455, 430–433 (1988). (7) L. Dyrskov, B. W. Strobel, B. Svensmark, and H. C. Hansen, Beta-thujaplicin: New quantitative CZE method and adsorption to goethite, J. Agric. Food Chem., 52, 1452–1457 (2004). (8) Y. Higashi, M. Sakata, and Y. Fujii, High-performance liquid chromatography with dual-wavelength ultraviolet detection for measurement of hinokitiol in personal care products, J. Cosmet. Sci., 60, 519– 525 (2009). (9) K. Imai, Analytical chemical studies on high-performance recognition and detection of bio-molecules in life, Yakugaku Zasshi, 123, 901–917 (2003). Table III Level of Hinokitiol in a Skin Lotion and Recovery of Spiked Hinokitiol Assay Amount (μg/ml) Recovery (%) Added (50.0 μg) Added (100 μg) Day 1 204 88.8 93.2 Day 2 180 94.1 98 Day 3 212 91.3 91.9 Day 4 192 92.2 94.4 Day 5 183 84.5 89.2 Average ± S.D. (R.S.D.) 194 ± 14 (7.2%, n = 5) 91.8 ± 3.7 (4.0%, n = 10)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)