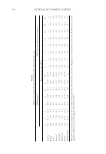
PHYSICOCHEMICAL PROPERTIES OF DELIPIDIZED HAIR 367 reveals greater hysteresis values in the case of delipidized hair indicating more resistance to release water. One should bear in mind that generation of DVS isotherms of hair fi bers is a kinetically governed process. For example, a study by Wortmann et al. (11) revealed no difference in the water sorption or desorption properties when comparing virgin, dam- aged, and hair treated with a cationic surfactant. More than likely, their experiments were carried for extended periods of time allowing full equilibration to be reached. Such ex- periments, when done properly, can take several weeks to complete for one sample. Often, shorter equilibration periods are used so that data may be obtained in a reasonable amount of time. One should also consider what types of equilibration time frames are reasonable in terms of the daily climatic experiences of a hair fi ber. SURFACE ENERGY ANALYSIS We used inverse gas chromatography surface energy analysis (iGC SEA) to probe the sur- face energy profi le of hair, which contains a dispersive ( ) γ d s and acid–base component ( ) γ AB s that contribute to the total surface energy ( ): γ T s γ = γ + γ T d AB s s s (1) The dispersive surface energy accounts for the London dispersion forces, van der Waals forces, and Liftschitz interactions, whereas the acid–base component takes into consider- ation acid–base and polar interactions. The dispersive surface energy analysis was per- formed by measuring the net retention volume VN (measured retention volume minus Figure 7. Stiffness ratio values for virgin and delipidized hair treated with various molecular weight samples of PVP.
JOURNAL OF COSMETIC SCIENCE 368 dead volume) for a series of alkane elutants (in this case, heptane, octane, nonane, and decane). For the analysis, the method of Dorris and Gray was applied. In this method, a plot of RTln(VN) vs the carbon number (of the alkanes) should produce a linear regres- sion. The dispersive component of the solid sample can then be determined from the slope of the regression. Overall, we observe that the dispersive surface energy is greater for virgin (~55 mJ/m2) than delipidized (~48 mJ/m2) hair at low surface coverage. The dispersive surface energy distribution is also more heterogenous for virgin hair. Such as result is consistent with expectations as the sample containing less lipid should interact less with the hydrocarbon probe samples in terms of van der Waals and other nonpolar interactions. In addition, because there should be a greater variety of lipid species on the surface of virgin hair, we expect a larger dispersive energy distribution. The acid–base component of the total surface energy (also called specifi c surface energy) is obtained via iGC SEA by fi rst measuring the specifi c free energies of desorption for dif- ferent polar probe molecules, ΔGSP. These values were determined by measuring the re- tention volume of polar probe molecules (ethanol, acetone, ethyl acetate, and chloroform) on the hair samples. In the polarization approach, the ΔGSP values are determined from a plot of RTln(VN) vs the molar deformation polarization of the probes, PD. Points repre- senting a polar probe are located above the alkane straight line in the RTln(VN) vs PD plot. The distance to the straight line is equal to the specifi c component of the free energy of desorption, ΔGSP. From the ΔG values, one can calculate acid–base numbers which are related to the specifi c surface energy. Although the acid–base surface energy values are similar for both hair types—ranging from ~5.75 to 6.25 mJ/m2—the distribution is broader for the delipidized sample. This could indicate that once the layer of lipids is removed from hair surface, the probes experience a greater variety of polar interactions due to the underlying exposed protein side chains. In addition, Guttman (ka and kb) values were calculated from the specifi c energy data. ka and kb, respectively, provide information about the electron-donating and electron-accepting Figure 8. Sorption and desorption isotherms for virgin and delipidized hair.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)