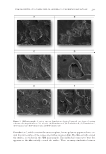
JOURNAL OF COSMETIC SCIENCE 204 because of convenience of application, especially on children. The use of polymers in such formulations has become a standard practice as polymers impart water resistance and contribute to boosting the sun protection factor (SPF) of such preparations. In many instances, polymers affect sensorial properties of the formulations as well. During the past several years, most of the research conducted on the use of polymers in sunscreen formulations has been centered around developing new methodologies for testing in vitro SPF and water resistance (1–3). Recently, the effect of polymers on sensorial attributes was also investigated (4). The mechanism by which poly- mers affect waterproofi ng of sunscreens was described by Prettypaul and Fares several years ago (5). Although the authors described mechanistic information on the forma- tion of a polymeric fi lm, additional information on the fi lm properties needed to be investigated. In this study, we developed a direct method by which one can visualize polymer fi lms after they are sprayed onto stratum corneum sheets. The method allows us to study the interaction of various polymer combinations on fi lm surfaces and their ability to form a continuous fi lm on the surface. We used vapor transmission data for such fi lms to under- stand the breathability of polymer mixtures on the skin and how it is related to in vitro water resistance. MATERIALS AND METHODS In this study, we introduce a methodological approach for investigating sunscreen fi lm formation on the skin. Specifi cally, we probed the interactions of the primary fi lm former, VA/butyl maleate/isobornyl acrylate copolymer, with two commonly used fi lm formers in spray formulations, namely, acrylates/dimethicone and hydroxypropyl cellulose. All formulations used in this study are displayed in Table I. The chassis developed was quite simple and contains typical ultraviolet A (UVA) and ultraviolet B (UVB) sun- screens, functional polymers, and alcohol (the diluent). The formulations were then aero- solized as described in the following paragraph. MATERIALS Butyl methoxydibenzoylmethane (Escalol™ 517), benzophenone-3 (Escalol™ 567), ho- mosalate (Escalol™ HMS), ethylhexyl salicylate (Escalol™ 587), octocrylene (Escalol™ 597), isostearyl neopentanoate (Ceraphyl™ 375), VA/butyl maleate/isobornyl acrylate copolymer (Advantage™ Plus), and hydroxypropyl cellulose (Klucel™ G CS) were sup- plied by Ashland Specialty Ingredients G.P. (Covington, KY). Acrylates/dimethicone copolymer (KP 545L) was obtained from Shin-Etsu Chemicals (Tokyo, Japan). Alcohol, SD 40-B (200 proof), was provided by Pride Chemical Solutions (Holtsville, NY) and butylene glycol was purchased from Thermo Fisher Scientifi c (Waltham, MA). In addi- tion, tests were conducted with a commercial SPF 30 aerosol sunscreen formulation con- taining the following ingredients: avobenzone, octocrylene, oxybenzone, SD alcohol 40-B, isobutane, dimethicone, tocopherol, ascorbyl palmitate, VA/butyl maleate, isobornyl acrylate copolymer, and fragrance.
FILM PROPERTIES OF POLYMERS USED IN ANHYDROUS SUNSCREEN FORMULATIONS 205 FORMULATION PROCEDURE In all formulations, phases A and B were weighed separately in different beakers. Phase B was heated to 50°C until all crystals were dissolved and then brought back to room tem- perature. Phase A was added to phase B and then ingredients of phase C were added to their respective formulations. Formulations were fi lled into an aluminum can and aero- solized with 30% isobutane (A-31). The actuator was a Moritz twist-to-lock with a Misty 0.025 insert from Aptar (Crystal Lake, IL). VAPOR FLUX METHODOLOGY Evaporimeter measurements were performed using an AquaFlux Model AF200 (Biox Systems, Ltd., London, UK) on pig skin that was placed in a Franz diffusion cell assem- bly. The evaporimeter was fi tted with an adapter to fi t directly onto the Franz diffusion cells. Dermatomed pig skin was cut into 400 mm2 sections and placed over the donor chamber. Test samples were applied on the pig skin in quantities of 2 mg/cm2 using an analytical balance and then spread evenly with a fi nger cot. The receptor fl uid was fi lled with deionized water and the temperature of the skin and the receptor fl uid was kept constant at 34°C using a circulating water bath. Skin was equilibrated for 15 min at that temperature before sample application. Data collection (1 point/s) took place over a period of 60 min to monitor the variations in vapor transport as a function of time. TAPE STRIPPING PROTOCOL Products were sprayed onto a stratum corneum layer that was formed on a D-Squame disc. Standard 22 mm D-Squame discs (CuDerm Corporation, Dallas, TX) were used to Table I Fo rmulations Tested Ingredients Formulations A B C D E F Phase A Alcohol, SD 40-B (200 proof) 93.00 94.00 54.00 52.00 51.00 51.80 Butylene glycol 2.00 2.00 2.00 2.00 2.00 2.00 Phase B Butyl methoxydibenzoylmethane 3.00 3.00 3.00 3.00 Benzophenone-3 6.00 6.00 6.00 6.00 Homosalate 15.00 15.00 15.00 15.00 Ethylhexyl salicylate 5.00 5.00 5.00 5.00 Octocrylene 10.00 10.00 10.00 10.00 Isostearyl neopentanoate 5.00 5.00 5.00 5.00 5.00 Phase C VA/butyl maleate/isobornyl acrylate copolymer (and) alcohol (50% w/w solution in alcohol) 4.00 2.00 2.00 2.00 Hydroxypropyl cellulose 0.20 Acrylates/dimethicone copolymer (and) dimethicone (40/60% w/w) 1.00 Total 100.00 100.00 100.00 100.00 100.00 100.00
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)