JOURNAL OF COSMETIC SCIENCE 300 Based on their mechanism of action, topical peptides are classifi ed into four categories: signal peptides, enzyme-inhibitor peptides, neurotransmitter-affecting peptides, and car- rier peptides (1–4). Lys–Thr–Thr–Lys–Ser (KTTKS) (see Figure 1) is a signal peptide discovered by Katayama et al. in 1993. They demonstrated that extracellular matrix biosynthesis in human fetal lung fi broblasts was stimulated by KTTKS as a subfragment from C-peptides of type I procollagen, which is able to increase dermal remodeling (5). Despit e its high antiaging potential, this peptide minimally penetrates the skin. One strategy used to overcome this problem is conjugation to a lipophilic compound such as palmitic acid (a long-chain fatty acid containing 16 carbon atoms) (6). Palmitoyl-KTTKS (Pal-KTTKS) (see Figure 1) (brand name MatrixylTM, Sederma Inc., Le Perray-en-Yvelines, France) is available on the global market in antiaging formulations. Pal-KTTKS demon- strated improved effects on reduction of skin wrinkles in a 12-week, double-blind, placebo- controlled, clinical study on photoaged human facial skin (7). Fu et al. (8) demonstrated that niacinamide/Pal-KTTKS/retinyl propionate products had greater effect on improve- ment of wrinkle appearance than a 0.02% tretinoin product. Unfortu nately, only few preformulation studies exist on them. Lack of information about physicochemical proper- ties of these compounds leads to diffi culty in their formulation. Here, K TTKS and Pal-KTTKS were synthesized, and after confi rmation by mass spec- troscopy, physicochemical properties of both peptides and, therefore, the effects of covalent attachment of palmitic acid on KTTKS properties were investigated. To achieve these goals, ultra violet (UV) absorption ability, structure, morphology, birefringence, thermal Figure 1. Chemical stru c ture of KTTKS (A) and Pal-KTTKS (B).
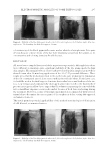
PREFORMULATION STUDIES OF PEPTIDES KTTKS AND PAL-KTTKS 301 behavior, aqueous solubility, partition coeffi cient, surface activity, critical micelle concen- tration (CMC), and aqueous stabilities were evaluated. The results should be useful for formulation development and also will provide information on palmitic acid conjugation of peptides, possibly useful for peptides other than KTTKS. MATERIA L AND METHOD MATERIA LS 2-chlor otrityl chloride resin (loading capacity of 1.2 mmol/g), Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Lys(Boc)-OH, and 2-(7Aza-1H-benzotriazol-1-yl)-1,1,3,3- tetramethyluronium hexafluorophosphate (HATU) were provided by GL Biochem (Shanghai, China). Trifl uoroacetic acid and piperidine were obtained from Exir GmbH (Wien, Austria). Ninhydrin was purchased from BDH Chemicals (Lutterworth, England). Isopropyl alcohol was supplied by ChemLab (Zedelgem, Belgium). Other solvents and reagents applied in the peptide synthesis process were purchased from Sigma-Aldrich (Dorset, United Kingdom) or Merck (Gernsheim, Germany). All materials were used without further purifi cation. METHODS Peptide synthesis and characterization. KTTKS an d Pal-KTTKS were synthesized by solid- phase peptide synthesis (SPPS) using the standard fl uorenylmethyloxycarbonyl (Fmoc) strategy in a manual glass reaction vessel (9). The 2-chlorotrityl chloride resin was ap- plied as an insoluble support, and to protect side chain reaction during the process, amino acids containing tert-butyloxycarbonyl (Boc) or tert-butyl side-chain protecting groups were used. After synthesis, confi rmation of peptide integrity was accomplished by mass spectroscopy using an Agilent 6410 Triple Quad LC/MS (Agilent, Santa Clara, CA) with an electrospray ionization interface. Determin ation of UV absorbance. Aqueous solutions of KTTKS and Pal-KTTKS were pre- pared individually. First, UV absorbance was set to zero with deionized water, and the solvent of peptide solution was used as the blank. UV absorbance of solutions was then measured over the wavelength range of 190–400 nm by a Cecil 2021 UV-Visible spectro- photometer (Cecil Instrument Services, Cambridge, United Kingdom) using a quartz cuvette with a 1-cm path length. Evaluati on of particle morphology by SEM. The morp hology of KTTKS and Pal-KTTKS was analyzed by SU3500 scanning electron microscopy (Hitachi Ltd., Tokyo, Japan) at an ac- celerating voltage of 15 kV. Peptide powders were mounted on a stub of metal with ad- hesive, coated with gold, and then analyzed by SEM. Evaluati on of birefringence under polarized light microscopy. Birefrin gence was examined using a CETI Magnum binocular compound microscope (Medline Scientifi c, Oxford, United Kingdom) with a total magnifi cation of 100×. The glass slide of each peptide powder was prepared and observed under cross-polarized light at ambient temperature. Structure evaluation by X-ray diffraction (XRD). XRD patte rns of KTTKS, Pal-KTTKS, and palmitic acid were measured using an X-ray diffractometer (X’Pert Pro MPD,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)