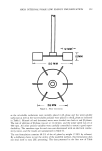
358 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS time as the result of adopting LEE can be very substantial. In some cases, the reduction of cooling requirements can be so great that the use of a refrigerated system can be entirely eliminated in designing a new plant (2). Previous experimental work indicated that when correctly processed, the quality of the emulsions made with LEE was virtually indistinguishable from that of the emulsions made with conventional hot processing (3). Although it is clear that the greater the ratio of tx to [3, the greater will be the energy conservation, there is a practical limit to the size of the alpha phase because of potential, irreversible phase inversion as pointed out in an earlier work (4). Uncontrolled phase inversions and the resulting reduction in emulsion stability or coarsening of the emulsion texture are also the main reasons why it has been difficult to apply LEE on emulsions containing relatively high solids or emulsions having internal phases exceeding 30% of the total volume. Emulsions stabilized with nonionic surfac- tants may undergo phase inversion at temperatures near the phase inversion temperature (PIT). LEE processing may lead to the formation of a coarse emulsion if phase inversion is not controlled (5). On the other hand, when the key variables are properly controlled, LEE can produce very fine emulsions having significantly smaller mean droplet sizes than the emulsions prepared with a conventional high-energy method (6). The purpose of this work was to test a new technique of double withholding which was designed to expand the utility of LEE beyond low-internal phase O/W emulsions and also to allow LEE processing in a relatively high tx range. In the original LEE method, only a portion of either the external or internal phase is withheld during the initial emulsification process. The new technique now involves withholding portions of both phases. To avoid confusion, the withheld, unheated portions of the internal and external phases will be designated as ix, phase and tx e phase respectively. Similarly, the heated portions of the internal and external phases will be respectively referred to as [3i phase and [•e phase. In the original method, emulsification is carried out in two stages, including a con- centrate preparation stage and a dilution stage as indicated below: Original LEE First Stage: Concentrate Preparation Internal Phase (heated) + [•e • EC (Emulsion Concentrate) Second Stage: Concentrate Dilution EC + tx e -• Ef (Final Emulsion) The new LEE technique to be introduced here involves withholding of both txi and tx e and hence requires a three-step operation as indicated below: New LEE (Double Withholding) First Stage: Concentrate Preparation [•i q- [•e • El (First Emulsion Concentrate) Second Stage: tXe-Phase Addition E1 q- O•e '• E2 (Second Emulsion Concentrate) Third Stage: txi-Phase Addition E 2 + tx i --- Ef (Final Emulsion) The advantage of the double withholding technique can be readily seen from Figure 1. In this illustration, the internal phase volume is approximately equal to one third
HIGH INTERNAL PHASE LOW ENERGY EMULSIFICATION 359 EXTERNAL PHASE -x• CASEfA} INTERNAL PHASE --• ! ...... (A)- ......... (B}--- L ! =0.33 .•e: 0.67 CASE(B] C•.= 0.67 0.5• EMULSION Figure 1. Comparison of two double withholding LEE processes. In both cases internal phase equals one third of external phase oq and o• e are withheld internal and external phases respectively [3, and IBe are heated internal and external phases respectively. Case B is more energy efficient due to a larger withheld external phase %. Splitting of the internal phase into oq and [3, and using only [3, for the initial emulsi- fication reduce the danger of phase inversion. of the external phase volume. For LEE processing, the external phase is predivided into withheld OL e phase and heated [•e phase. If the division of the external phase were made at O•e/lBe = O. 33/0.67 as in the Case (A), indicated by a dashed line, the volume of [•e phase would be significantly larger than the total internal phase and generally there would be no great danger of phase inversion. To achieve even greater energy conser- vation, the division of the external phase may be made at a higher o•/[3c ratio. In Case (B) shown with a dotted line, the OLJ[• e ratio is chosen at 0.67/0.33. This means that the [3c phase is now roughly equal to the total volume of the internal phase. From phase volume considerations, there is now a greater danger of unintended phase inver- sion during the first stage of the emulsion concentrate preparation. One way to minimize the chance of unintended phase inversion is to add the external phase liquid slowly to the internal phase liquid. In a plant-scale operation, however, such a method is not only time-consuming, but may be unreliable. Controlling the initial surfactant location as proposed by Lin and Lambrechts may be useful in borderline cases, but it is not a sure way to control phase inversion (7). A better method proposed here is to divide the internal phase into oq and 13i. If, for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)