360 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS example, the internal phase were subdivided into two equal parts as shown in Figure 1, and if only the •i phase were used to prepare the emulsion concentrate, the ratio of the external phase to the internal phase volume for the concentrate would now be about two instead of one for Case (B). For Case (A), the ratio would now be approximately four. The substantial increase in the ratio of the external phase volume to the internal phase volume in the first-stage concentrate preparation will greatly reduce the chance of phase inversion during this stage. Since the second stage operation involves only the addition of an external-phase fluid (o• e phase) there is little danger of phase inversion. Although the third stage operation requires addition of the remaining internal phase (oq phase) there is no great danger of phase inversion since an additional amount of the external-phase fluid was added to the bulk in the second stage operation. Hence, the new double-withholding procedure effectively reduces the danger of irreversible phase inversion during the manufacturing process and allows LEE processing at relatively high o•/• ratios to achieve substantial energy conservation. EXPERIMENTAL Experiments were carried out in 250-ml glass beakers with no baffles. A six-blade turbine mixer driven by a high-torque, variable speed motor was used to prepare the emulsions. The dimensions of the turbines are indicated in Figure 2. Since the rate of phase addition was found to affect phase inversion, the rates of adding the o• e and oq phases were carefully controlled by introducing the fluid through a buret with a constant orifice. Other experimental details, including heating and combining the phases, were similar to those of the procedure used in previous reports (3-5). Droplet size distri- butions were obtained photographically. The mean droplet diameter represents an arith- metic average of approximately 200 droplets. The •e and •i phases were heated to 75øC before addition, and the o• e and oq phases were kept at 26øC before addition. RESULTS AND DISCUSSION EFFECTS OF % AND % According to an earlier theory advanced by Ostwald, emulsion phase inversion occurs when the internal phase volume exceeds 74.02% of the total volume (8). However, this argument is based on closely packed uniform spheres, which does not necessarily represent a real emulsion. For non-uniform droplets, the critical volume fraction of the internal phase, 4), may far exceed the 0.7402 limit without phase inversion. In commercial processing of emulsions, however, phase inversion depends not only on phase volume but also on many complex factors, including surfactant HLB, processing temperature, method of preparation, mixer geometry, mixer speed, and the rate of phase combination. Generally, the phase-inversion problem becomes more troublesome to control in a plant operation when 4) values exceed 0.4. For our experiments, we selected O/W formulations having approximately 50% internal phase volumes. One example of such a formulation is in Table I. Using the above formulation, one hundred emulsions were prepared with o• e and oq values ranging from 0 to 0.9. In this series of experiments, all waxy substances as well
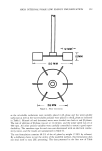
HIGH INTERNAL PHASE LOW ENERGY EMULSIFICATION 361 50MM ---.I r2 MM I--'- -t- IOMM •-• •6 MM •--4 as the oil-soluble surfactants were initially placed in IBi phase and the water-soluble surfactant as well as the water-soluble polymer were placed in the IBe phase as indicated in Table I. Mineral oil and deionized water were divided into both o• and [• phases. The rate of addition of [3 phase was set at 14 ml/min. and the mixer speed was set at 400 rpm. All other process variables were carefully controlled to assure a good repro- ducibility. The emulsion type for each run was determined with an electrical conduc- tivity tester, and the results are summarized in Table II. The test formulation contains 48.5% of the oil phase by weight (50% by volume). By a deliberate choice to test the utility of the modified method, this formulation does not lend itself to easy LEE processing. The data presented in the first row of Table
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)