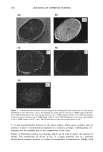
ADSORPTION OF POLYMER ONTO HAIR 277 (a) . H:C CH: n (b) Figure 1. a: Structure of the polyquaternium-6 polymer. b: Structure of the phosphorus-labeled polyquater- nium-6 polymer. The molar ratio of n:m is approximately 99:1. For polymer synthesis see text. INSTRUMENTATION Environmental SEM. Environmental SEM (ESEM) permits high-resolution imaging of fully hydrated materials. ESEM offers all the capabilities of conventional SEM, but a differential pumping system enables specimens to be maintained in a gaseous environ- ment to around 20-torr pressure (11). Secondary electron imaging exploits the ionized gas in the chamber by using proportional gas amplification of the SE signal (12). The ionized chamber gas also dissipates charge buildup on the specimen. Water vapor is a convenient and efficient imaging gas. By balancing chamber pressure and specimen temperature along the 100% RH isobar, specimens may be maintained in a fully
278 JOURNAL OF COSMETIC SCIENCE hydrated state for long periods of time. Resolution is comparable to a conventional SEM (-5 nm with a tungsten gun). Techniques like energy dispersive spectroscopy (EDS) are routinely employed, but there are special considerations due to the gas in the chamber (13,14). There is little literature on ESEM applications to human hair, but related studies include the study of wool (15) and the development of detergents and personal products (16). In this work a Philips-Electroscan ESEM 2020 was used with a PGT IMIX EDS system. Hair was clamped in a special metal stub designed to hold hair strands fiat and to allow reproducible positioning in the ESEM. In this way, individual strands could be exam- ined before and after treatment with conditioning polymers. The stub was temperature- controlled via a Peltier stage. Specimens were typically imaged at -5øC and chamber pressure -5 Torr to maintain approximately 100% RH. X-ray photoelectron spectroscopy (XPS). XPS analyzes the near-surface of materials with analysis depths up to 50 •. It can give quantitative chemical information as well as oxidation and structural environments on all elements apart from hydrogen and helium. Soft X-rays, of energy hv, excite valence electrons from valence and core orbitals of surface atoms. This is shown in Figure 2. The kinetic energy of the ejected electron, EK, is measured by an electron energy analyzer. These photoelectrons have energies according to the relationship (17) E B -- hv - E K - where (I) is the work function of the spectrometer. E B is the binding energy of the photoelectron to the parent atom, and this can be calculated from the other measured and known values. XPS spectra are traditionally plotted as EB vs photoelectron intensity. XPS peaks can then be identified using tabulated binding energy values from XPS handbooks yielding information on chemical composition and bonding environments. Tresses of untreated and polymer-treated hair were clamped into separate metal stages such that a mesh of hair at least 5 mm by 5 mm could be studied. XPS spectra were acquired using a Fisons ESCAscope photoelectron spectrometer with dual anode X-ray 2P3/2 2Pl/2 2S llllllllllll •llllmml Vacuum level core Initial state Final state E K E B hD Figure 2. Emission of a ls photoelectron after X-ray beam of energy hv has interacted with atom. E B is electron binding energy and E K the kinetic energy after leaving vacuum level.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)