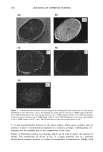
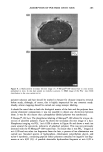
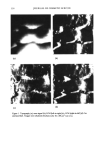
j. Cosmet. Sci., 51,275-287 (September/October 2000) Advancements in spectroscopic and microscopic techniques for investigating the adsorption of conditioning polymers onto human hair JAMES S. DALTON, GEOFFREY C. ALLEN, PETER J. HEARD, KEITH R. HALLAM, NICK J. ELTON, MATTHEW J. WALKER, and GARY MATZ, Interface Analysis Centre, University of Bristol, 121 St. Michaels Hill, Bristol BS2 8BS, UK (J.S.D., G.C.A., P.J.H., K.R.H.), Imerys Central Research, Par Moor Laboratories, St. Austdl, Cornwall PL24 2SQ, UK (N.J.E, M.J.W.), and Calgon Corporation, P.O. Box 1346, Pittsburgh, PA 15230 (G.M.). Accepted for publication August 11, 2000. Synopsis Adsorption of the polyquaternium-6 (Merquat ©- 100) conditioning polymer onto bleached human hair has been investigated using environmental scanning electron microscopy (ESEM), X-ray photoelectron spec- troscopy (XPS), and secondary ion mass spectrometry (SIMS). ESEM is not limited to a high-vacuum environment, and therefore hair morphology could be studied under ambient and hydrated conditions. XPS gave elemental analysis on the surface of the hair, in addition to information on the chemical environment of the surface atoms. SIMS can produce high-resolution ion distribution images on the hair's surface. Both XPS and SIMS detected carbon, nitrogen, oxygen, and sulfur on the surface of the untreated bleached hair, all of which were attributed to the biological matrix. Silicon was also detected and its presence was attributed to either a previous silicone cosmetic application or to surface contamination. Due to the similar elemental composition of hair and Merquat-100, treatment of the hair with a phosphorus-labeled polyquaternium-6 cationic conditioning polymer was also investigated. XPS, with sensitivity of 1000 parts per million (ppm), could not detect phosphorus present from any adsorbed polymer, but SIMS, with 1-10 ppm sensitivity, allowed high-resolution images to be obtained that illustrated the adsorption of polymer onto the hair's surface. INTRODUCTION Due to the increasing complexity of personal care formulations and strong market forces, there is now a greater necessity than ever for manufacturers to substantiate claims concerning their products. Therefore, there is a current need for further development of analytical techniques capable of giving biological and chemical information on both the hair's surface and adsorbed cosmetic molecules such as polymers. The aim of this paper is to demonstrate how techniques such as X-ray photoelectron spectroscopy (XPS), secondary ion mass spectrometry (SIMS), and environmental scanning electron micros- 275
276 JOURNAL OF COSMETIC SCIENCE copy (ESEM) can be exploited to this end. These techniques have the advantage over many indirect and macroscopic tests that give little information on hair morphology and the presence of specific adsorbates such as polymers. Quaternized cationic polymers have a high affinity for the hair's surface due to the hair's negative charge at normal pH and are typically used as conditioners due to their high cationic charge density. Generally, hair conditioning involves some interfacial alteration in the protein matrix of the hair. There is a wealth of literature covering electron microscopy studies of hair (1). Studies include ESEM, which, unlike traditional SEM, does not require a high vacuum and can allow the hair to be studied in an ambient and hydrated environment (2). More recently, attention has been paid to hair structure and morphology using scanning probe and atomic force microscopy (SPM and AFM), allowing the hair to be imaged at varying pH and hydration levels (3-6). SPM has also been instrumental in detecting cationic poly- mer adsorption onto hair, these polymers adsorbing strongly at normal pH due to high cationic charge density, an essential property for hair conditioning (7,8). There is little literature on spectroscopic studies of hair however, electron energy loss spectroscopy (EELS) has been used to map the sulfur distribution in human hair, giving information on the distribution and abundance of keratin bonds (9). In addition, SIMS imaging has recently been proposed by Gillen et •l. as a promising technique for mapping distributions of certain ions in hair such as Ba +, K +, and C2H3 + after doping hair in various inorganic and organic solutions. Their recent work gives some good examples (10). With the emphasis on cosmetics, this paper shows how a variety of techniques can be used to investigate the presence of adsorbed species onto biological materials such as hair. MATERIALS AND METHODS GENERAL HAIR TREATMENT Bleached hair samples were purchased from DeMeo Brothers, New York. The bleached hair was studied both untreated, by simply rinsing the sample in distilled water and allowing to dry naturally, and treated, as outlined below. Treatment was carried out using polyquaternium-6, a cationic conditioning polymer, [poly(dimethyldiallyl ammonium chloride), Merquat©-100, 40% active, Calgon Corpo- ration, for polymer composition (see Figure l a)] by soaking in a 0.1% w/w aqueous solution (18Mf• deionized water) for two hours and then rinsing in running deionized water for two minutes. The tress was then left to dry naturally for 24 hours. To enable clearer detection of the polymer adsorption, and to differentiate the polymer from the hair with greater distinction, some tresses were treated with a phosphorus- labeled polyquat-6 derivative. This phosphorus-labeled Merquat © 100 was prepared by polymerizing dimethyldiallylammonium chloride in the presence of hypophosphorous acid as a chain regulator. The structure is given in Figure lb. For distinction, the commercially available Merquat©-100 will be referred to as N-Merquat©-100, and the synthesized phosphorus-labeled as P-Merquat- 100.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)