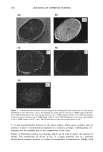
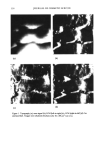
ADSORPTION OF POLYMER ONTO HAIR 279 sources (Mg/A1) and a concentric hemispherical electron energy analyzer. Data acquisi- tion and manipulation was carried out using microprocessor units with VGS software. During acquisition of spectra the pressure in the main chamber was maintained at 8 x 10 -9 torr to ensure a clean sample surface. Charge referencing was carried out against adventitous hydrocarbon (Cls 284.6 eV) (17) from pump oil contamination. Secondary ion mass s[•ectrometry (SI&IS). SIMS allows a mass spectrum of elemental and molecular species present in the surface to be obtained (18). A highly focused ion beam penetrates the surface under study, sputtering atoms and ions in the uppermost surface layer, which are then analyzed according to their mass/charge (m/z) ratio by a mass spectrometer. In a manner similar to SEM imaging, high-resolution SIMS images were obtained by raster scanning the beam over the desired area on the hair's surface and detecting locations of sputtered ions having a prefixed m/z value. However, in contrast to XPS, SIMS is a surface-destructive technique due to the sputtering or erosion of the surface atoms, and therefore acquisition times for spectra and images were kept to a minimum. The SIMS spectra and images were obtained using an in-house instrument comprised of an electronically variable aperture-type gallium ion gun (FEI SD Gallium LMIS EVA focusing column) fitted to a double-focusing magnetic-sector mass analyzer (Vacuum Generators model 7035). An Everhart-Thornley electron detector was used for acquisi- tion of secondary electron images. For secondary ion analysis, a lnA, 25keV impact Ga + microbeam was used, which allowed an image resolution of 300 nm. The upper mass limit was configured at 100 daltons (D), and the instrument was calibrated using values of 68.926 and 70.925 D for the backscattered Ga + ions. Analysis was carried out using the "Dayta" software package (19) running under Windows 95. For obtaining spectra of the hair's outer surface (epicuticle), 10-mm hair lengths were mounted over an aluminium stub using silver dag adhesive at either ends of the hair. It was essential that the hair made good contact with the stub throughout its length, and so an aluminium mesh was placed over the sample to essentially clamp the hair and enable it to lie flat. Due to problems of imaging hair lengths, which will be outlined later, SIMS images were obtained on hair cross sections. Here 10-mm-length samples of both N- and P-Merquat-100-treated hair were embedded in separate blocks of Agar 100 epoxy resin and then microtomed with a diamond knife. Due to the insulating nature of the resin and hair, the blocks were gold-coated for analysis to eliminate charging. Careful coating of the hair under high vacuum would not affect the elemental distri- bution, and the metal layer was etched away before analysis using the lnA 25keV Ga + microbeam. RESULTS AND DISCUSSION ESEM Figure 3a shows an ESEM image of virgin bleached hair. Figure 3b shows hair from the same batch after exposure to pH 9.5 at 20.9øC. Some roughening and raising of the cuticle is visible. Figure 3c shows hair from the same batch after treatment with a 3% aqueous solution of Merquat-100 at pH 7. Although a little raising of the cuticle still seems to be present after treatment with Merquat-100, this may be expected, as the hair
280 JOURNAL OF COSMETIC SCIENCE (a) co) (½) Figure 3. a: Untreated virgin bleached hair. b: Virgin bleached hair exposed to aqueous environment at pH 9.5 and 29.9øC. Some raising of the cuticle is visible. c: Hair treated with 3% Merquat-100 at pH 7. Conditioning treatment is expected to smooth and lower the cuticle in damaged hair. was treated only once with the polymer solution to allow fair comparison to the other techniques. However, the many images obtained from both untreated and treat- ed hair showed less roughening on the conditioned cuticle compared to the untreated. While ESEM is useful for revealing surface topography of the hair, it was not useful for determining the distribution of Merquat over the surface owing to lack of topo- graphic or Z contrast (except at unreasonably high polymer doses). Hair treated with N-Merquat and P-Merquat was examined using EDS. The elemental composi- tion of N-Merquat was not sufficiently different from that of the hair to allow EDS mapping of the polymer distribution. In principle, the theoretical level of P in the P-Merquat ought to be high enough to allow detection by EDS. However, in practice, the concentration of P over the surface of P-Merquat-treated hair was below the detec- tion limit, and this approach was abandoned in favour of SIMS and XPS. Beam damage can be a problem in ESEM, especially with hydrated biological materials during EDS (14). In this study, beam damage to the hair did not appear to occur under normal operating conditions. XPS The XPS spectrum of untreated hair is given in Figure 4. This shows the presence of carbon, nitrogen, oxygen, sulphur, and silicon from the outer surface of the epicuticle. The first four elements were expected, as they form the biological matrix of the hair, the epicuticle being rich in the amino acid cysteine, which contains keratinized disulfide bonds that give the hair its strength (1). Silicon on the surface of hair can be explained by the presence of silicon polymers in many shampoo and conditioner formulations (20), and the hair may have been treated with these prior to study. In addition, it is commonly known that silicones contaminate the surface of most materials, in particular polymers (21), which makes any surface study of silicon difficult. Peak fitting was carried out on regional elemental spectra to obtain information on the chemical environment. The carbon Is photoelectron spectra was resolved to two peaks, with the most intense due to both the hydrocarbon chains present in the protein and pump oil contamination. The less intense peak was due to the carbon from the carboxylic groups in the protein matrix. The nitrogen and oxygen Is peaks at 399.9 eV and 531.8 eV, respectively, were char- acteristic of the organic biological matrix formed from the amino acids (22). For the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)