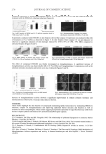
TREHALOSE IN HAIR CARE 239 Since trehalose is a sugar, it is tempting to assume that the non-fl uffi ng of the hair array could be due to the stickiness of the sugar, increasing tack and adhesiveness on the surface and holding the array in place even at high humidity. Here this is partly prevented by ensuring that the switches are combed at least fi ve times after the styling process to remove any surface “bonds” or “welds” forming and holding the array together. Moreover, after high-humidity, tactile tests show that there is a very small crisp/dry coating on the surface, suggesting possibly the presence of the trehalose dihydrate crystal. Apart from sugar stickiness, another possibility could be the difference in diffusion of water in fi bers treated with trehalose and heat. The adsorption isotherms shown by the DVS curves in Figure 3 enable calculation of diffusion coeffi cients using simplifi ed methods (19). It is found that there is no difference in the diffusion coeffi cients between control heat-treated and trehalose heat-treated hair, though both are signifi cantly smaller than with normal (non-heat-treated) control hair. In the literature the remarkable properties of trehalose in protecting living cells against extreme desiccation, etc., is thought to occur because trehalose works as a water replacement molecule or is a vitrifi cation agent in the dry state (20–28). Here a simple moisture uptake of the glassy form of trehalose seems to correlate well with the effect seen in hair. At normal room temperature the glass transition relative humidity of hair (RHg) is around 65–70% RH. Above this RHg, water uptake in hair increases rapidly, hair is plasticized, and style loss is accelerated. The effect of trehalose glass taking up water, however small, seems to increase the aging of the hair polymer, giving rise to preservation of straight style and consequently the anti-humidity benefi t. Further evidence of the effect of trehalose was seen on switches that were repeatedly subjected to heat straightening and high-humidity exposure without any wash in between, but with a mild rinse by spritzing with water from a wash bottle. Switches originally treated with trehalose continued to show low fl uffi ng at high humidity compared to the control for about three cycles. Here we think that in the trehalose-treated switches during the styling and high-humidity cycle, trehalose changes forms from glass to crystal to glass again to give rise to continued straight style longevity. A complete rinse or a full washing process results in the loss of much of the continued straight style benefi ts. A likely scenario for the mechanism of action for trehalose- and heat-treated hair could be the following. Trehalose is distributed at different parts of the hair composite from solu- tion, and when subjected to the straightening procedure with high-temperature irons, it Figure 7. Left: After styling with straighteners at ~20°C and 60% RH. Right: After changing the condi- tions in the room to high humidity at ~30°C and 80% RH. In both pictures the fi rst and the last switches are treated with water. All switches in the middle are treated with 2% trehalose solution. None of the switches shows the anti-humidity benefi t.
JOURNAL OF COSMETIC SCIENCE 240 produces trehalose glasses in situ at their current locations. The trehalose glasses formed pick up water as the humidity rises and thus reduce the amount of moisture available to disturb the style. Furthermore, the trehalose glasses may act as pore blockers and steri- cally hinder water from reaching certain parts of the hair. Finally, molecular trehalose may itself stabilize the styled confi guration through effects related to hair protein bind- ing (28) and/or structuring water (29). The striking anti-humidity benefi ts as demonstrated in this paper may be a result of one or more mechanisms described above. More work needs to be done to completely resolve the mechanism of action. In summary, here we see a macroscopic anti-humidity benefi t at the hair array level that seems intimately related to the water uptake and solid state polymorphism of trehalose. Clearly, glassy trehalose regulates moisture in the fi ber and affects the viscoelastic behavior and subsequent aging characteristics of the hair polymer in some way that, though not fully understood, seems to be related to the water uptake properties of this form of trehalose. We think that the presence of the trehalose glass and its ability to regulate moisture in the hair is the major contributing factor for the anti-humidity effect. CONCLUSION Hair treated with trehalose and heat-straightened shows long-lasting hair style reten- tion even at high humidity compared to hair treated with water, provided that the conditions at the style creation stage are suitable, i.e., low-humidity (50%) condi- tions. The formation of trehalose glasses in situ during the heat straightening process on hair treated with trehalose may be responsible for the anti-humidity effect seen. Treha- lose glasses regulate moisture in the fi ber at high humidity, giving longer-lasting style benefi ts. ACKNOWLEDGMENTS The authors thank Janet Cotterall for the initial switch work. REFERENCES (1) N. K. Jain and I. Roy, Effect of trehalose on protein structure, Protein Sci., 18, 24–36 (2009). (2) A. D. Elbein, Y. T. Pan, I. Patuszak, and D. Carroll, New insights on trehalose: A multifunctional mole- cule, Glycobiology, 13(4), 17R–27R (2003). (3) J. G. Streeter, Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation, J. Appl. Micro- biol., 95, 484–491 (2003). (4) N. Benaroudj, D. H. Lee, and A. L. Goldberg, Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals, J. Biol. Chem., 276(26), 24261–24267 (2001). (5) Y. Nie, J. J. de Pablo, and S. P. Palecek, Platelet cryopreservation using a trehalose and phosphate formulation, Biotech. Bioeng., 92(1), 79–90 (2005). (6) J. H. Crowe, L. M. Crowe, A. E. Oliver, N. M. Tsvetkova, W. F. Wolkers, and F. Tablin, The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state, Cryobiology, 43, 89–105 (2001). (7) W. F. Wolkers, N. J. Walker, Y. Tamari, F. Tablin, and J. H. Crowe, Towards a clinical application of freeze-dried human platelets, Cell Preserv. Technol., 1(3), 175–188 (2003). (8) J. K. Kaushik and R. Bhat, Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose, J Biol. Chem., 278(29), 26458– 26465 (2003).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)