J. Cosmet. Sci., 63, 243–254 ( July/August 2012) 243 A new chemical approach to optimize the in vitro SPF method on the HD6 PMMA plate S. MARGUERIE, M. PISSAVINI, A. BAUD, T. CARAYOL, and O. DOUCET, Lancaster–Coty, International Research & Development Center, Athos Palace, 2 Rue de la Lujernetta, MC 98000 Monaco. Accepted for publication December 21, 2011. Synopsis In a previous study, we demonstrated that control of the roughness of molded PMMA plates improves in vitro SPF reproducibility. However, in vitro/vivo deviations are still observed. Sunscreens show different behavior during spreading on the HD6 surface according to the formulation, re- sulting in a more or less homogenous distribution. The hydrophilic nature of HD6 appears to contribute signifi cantly during spreading. Two different sunscreens offering a homogenous and non-homogenous distri- bution were investigated to check if the interfacial tension between product and substrate has a real infl uence on the spreading quality. Using microscopic observations, we attempted to correlate the in vitro SPF results with the product’s spreading property. In order to reduce this interfacial tension, an HD6 pretreatment with an amphoteric surfactant, cocamido- propyl betain, was performed. In vitro SPF on “pretreated HD6” was examined using a cohort of 30 products. This pretreatment led to reliable results, demonstrating good association with the in vivo SPF. INTRODUCTION The challenge facing the cosmetics industry is to develop innovative, effi cient, sunscreen products that conform with European recommendations with a minimum of time and cost. In this regard, a reliable spectroscopic in vitro method is an essential tool. The advantages are well known. It is fast and relatively inexpensive, and moreover it offers complete spec- tral information on products. And importantly, there are no ethics problems (1–3). The EU recommendation on sun protection products, published in September 2006, recommends the use of in vitro methods. Although Colipa succeeded in establishing a UVA in vitro method (1), the in vitro SPF determination remains a challenge. Indeed, different working groups such as ISO, DGK, and even Colipa concentrated their efforts on devel- oping an in vitro reliable method with little success. All the ring tests performed led to the same conclusions: whatever the method used or the parameters chosen, inter-laboratory variability remains a major problem. The actual situation is the following: The in vitro SPF determination is accessible to every laborator, but the lack of control of the variables infl uencing the results can lead to poor results. The technique is very sensitive to different parameters (4). This may explain the
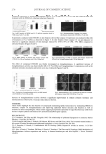
JOURNAL OF COSMETIC SCIENCE 244 deviation sometimes noted between the in vitro and in vivo results. Among the factors impacting on the in vitro SPF results, the most common are the device used to measure the transmission spectra, the amount of product applied, the application, the experience of the operator in the spreading process, and the substrate used to apply the product, or more precisely its roughness characteristics. One objective of Coty is to control more carefully these factors. Particular attention is paid to the substrate used for in vitro tests. In a previous study, we demonstrated how in vitro SPF reproducibility (5) can be improved by controlling the substrate’s surface micro- topography of the injected PMMA plates. As the roughness affects the SPF value, this factor must be fully controlled (6), but in spite of a constant PMMA roughness control, in vitro/vivo deviations are still observed. These experiments showed different product behavior during the product’s application on the surface of the HD6 PMMA, although the roughness remained constant. These phenomena clearly show that the physical properties of the PMMA molded plate are essential but are not the only substrate characteristics that play a key role in SPF results. In the course of our research on improving the in vivo/vitro correlation, the “physicochemical aspect” of the spreading process on the HD6 substrate was investi- gated. The cases of two different sunscreens offering a good and a bad adherence on the chosen substrate were compared. The objective was to study how the interfacial tension (I.T.) between product and HD6 molded plates can affect SPF results. The following study raises the possibility of modifying this I.T. by the use of a specifi c HD6 pretreatment with an amphoteric surfactant, cocamidopropyl betain. This paper describes how the amphoteric pretreatment led to a more universal in vitro SPF method suitable for every type of product tested, resulting in more reliable results. MATERIALS AND METHODS SUBSTRATE USED FOR IN VITRO SPF MEASUREMENT At the present time, PMMA is internationally recognized as a reliable substrate for in vitro sunscreen assay (1). The fi rst generation of PMMA plates was sandblasted, but in 2008 a new type of PMMA plate was introduced using injection molded manufacturing. Such PMMA plates offer a better batch-to-batch reproducibility. For this reason, the present study used the high-roughness molded PMMA plates supplied as Helioplate® HD6 (Helioscreen®, Creil, France). Each batch is validated by a control chart including ten roughness parameters (5). SUNSCREEN PRODUCTS SELECTED FOR IN VITRO SPF EVALUATION ON HD6 The in vitro SPF values of two different sunscreens, A and B, were investigated on the HD6 with exactly the same spreading procedure. Product A is an O/W emulsion, whereas product B is a polymer gel. Both products were selected for this study be- cause of their different in vitro behavior during spreading on HD6 as described in Table I. The second criterion of selection was the comparison of the in vivo to the in vitro SPF values.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)