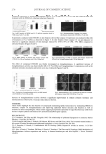
ANTI-TYROSINASE ACTIVITY OF ETHANOL FROM GRAPES 231 Figure 4. Effect of pure phenolic compounds on mushroom tyrosinase activity using L-dopa (0.33 mg/ml) as the substrate. Mushroom tyrosinase (44 units/ml) reacted at room temperature for 10 min. Symbols: - - (no inhibitor) - - (ellagic acid 0.03 mg/ml) -▲- (gallic acid 0.03 mg/ml) - - (kuromanin chloride 0.03 mg/ml) -×- (catechin hydrate 0.03 mg/ml). Gallic acid has been identifi ed as a tyrosinase inhibitor from many plants, and it inhibits the diphenolase activity of mushroom tyrosinase (18,19). Since the gallic acid content in RGG-SEE was about twice that in KG-SEE and gallic acid showed high anti-tyrosinase activity, we suggested that gallic acid was the main compound affecting the anti-tyrosinase activity in the ethanol extracts from grape seeds. CONCLUSION Grape seeds had higher anti-tyrosinase activity than grape peels because grape seeds had higher total phenolic compounds. The profi le of phenolic compounds was also an impor- tant factor affecting anti-tyrosinase activity. It was suggested that gallic acid was the main compound affecting the anti-tyrosinase activity in the ethanol extracts from grape seeds. The inhibitory mechanism of the ethanol extracts from the grape seeds was a mix-type inhibition. Since grape seeds were the potential source of the tyrosinase inhibitor, they might serve as a skin-lighting agent. ACKNOWLEDGMENT This study was supported by the National Science Council (NSC 97-2313-B-468- 002-MY3), Taiwan, ROC. Their fi nancial support is greatly appreciated. REFERENCES (1) S. Y. Seo, V. K. Sharma, and N. Sharma, Mushroom tyrosinase: Recent prospects, J. Agr. Food Chem., 51, 2837–2853 (2003). (2) A. Sánchez-Ferrer, J. N. Rodríguez-López, F. García-Cánovas, and F. García-Carmona, Tyrosinase: A comprehensive review of its mechanism, Biochim. Biophys. Acta, 1247, 1–11 (1995).
JOURNAL OF COSMETIC SCIENCE 232 (3) M. Friedman, Food browning and its prevention: An overview, J. Agr. Food Chem., 44, 631–653 (1996). (4) D. B. Mosher, M. A. Pathak, and T. B. Fitzpatrick, “Vitiligo: Ethology, Pathogenesis, Diagnosis and Treatment,” in Updates: Dermatology in General Medicine, T. B. Fitzpatrick, A. Z. Eisen, K. Wolff, I. M. Freedberg, and K. F. Austen. Eds. (McGraw-Hill, New York, 1983), pp. 373–398. (5) K. Maeda and M. Fukuda, In vitro effectiveness of several whitening cosmetic components in human melanocytes, J. Soc. Cosmet. Chem., 42, 361–368 (1991). (6) T. P. Dooley, Topical skin depigmentation agents: Current products and discovery of novel inhibitors of melanogenesis, J. Dermatol. Treat., 8, 275–279 (1997). (7) I. Kubo and I. Kinst-Hori, Tyrosinase inhibitors from anise oil, J. Agr. Food Chem., 46, 1268–1271 (1998). (8) N. H. Shin, S. Y. Ryu, E. J. Choi, S. H. Kang, I. M. Chang, K. R. Min, and Y. Kim, Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase, Biochem. Bioph. Res. Co., 243, 801–803 (1998). (9) I. Kubo and I. Kinst-Hori, Flavonols from saffron fl ower: Tyrosinase inhibitory activity and inhibition mechanism, J. Agr. Food Chem., 47, 4121–4125 (1999). (10) Q. X. Chen and I. Kubo, Kinetics of mushroom tyrosinase inhibition by quercetin, J. Agr. Food Chem., 50, 4108–4112 (2002). (11) J. K. No, D. Y. Soung, Y. J. Kim, K. H. Shim, Y. S. Jun, S. H. Rhee, T. Yokozawa, and H. Y. Chung, Inhibition of tyrosinase by green tea components, Life Sci., 65, 241–246 (1999). (12) A. Karioti, A. Protopapa, N. Megoulas, and H. Skaltsa, Identifi cation of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum, Bioorg. Med. Chem., 15, 2708–2714 (2007). (13) H. Y. Ding, H. C. Lin, and T. S. Chang, Tyrosinase inhibitors isolated from the roots of Paeonia suffru- ticosa, J. Cosmet. Sci., 60, 347–352 (2009). (14) M. Sato, N. Ramarathnam, Y. Suzuki, T. Ohkubo, M. Takeuchi, and H. Ochi, Varietal differences in the phenolic content and superoxide radical scavenging potential of wines from different sources, J. Agr. Food Chem., 44, 37–41 (1996). (15) Q. Li, Y. Jia, L. Xu, X. Wang, Z. Shen, Y. Liu, and K. Bi, Simultaneous determination of protocatechuic acid, syringin, chlorogenic acid, caffeic acid, liriodendrin and isofraxidin in Acanthopanax senticosus Harms by HPLC-DAD, Biol. Pharm. Bull., 29, 532–534 (2006). (16) T. O. C. Ndubizu, Relations of phenolic inhibitors to resistance of immature apple fruits to rot, J. Hor- tic. Sci., 51, 311–319 (1976). (17) W. Madani, S. Kermasha, and B. Bisakowaki, Inhibition of tyrosinase activity by a polyphenol esterase using selected phenolic substrates, Phytochemistry, 52, 1001–1008 (1999). (18) I. Kubo, I. Kinst-Hori, Y. Kubo, Y. Yamagiwa, T. Kamikawa, and H. Haraguchi, Molecular design of antibrowning agents, J. Agr. Food Chem., 48, 1393–1399 (2000). 19) I. Kubo, Q. X. Chen, and K. Nihei, Molecular design of antibrowning agents: Antioxidative tyrosinase inhibitors, Food Chem., 81, 241–247 (2003).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)