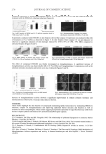
SAND RESISTANCE OF SUNSCREENS 257 At least 15 minutes after completion of the control standard application, each test site was divided into fi ve subsites, which were used for a progressive sequence of timed UV exposures, each of which was graduated incrementally by 15% over that of the previous exposure. All sites were evaluated between 22 and 24 hours after exposure by an evaluator who did not apply the control standard or sand or irradiate the subsites. The MED for each test site is that quantity of erythema effective energy, expressed in seconds, required to produce mild but defi nite erythema with clearly defi ned borders. Data were recorded onto case report forms. The SPF for each protected test site is that quantity of erythema effective energy, expressed in seconds, required to produce mild but defi nite erythema with clearly defi ned borders for the protected test site divided by that quantity of erythema effective energy, expressed in Table I Individual SPF Values Subject Skin type Age Gender Control standard Fine sand Medium sand All-purpose sand No. ID 1 51970 II 57 M 14.2 12.4 14.2 14.2 2 11701 II 32 M 18.8 18.8 18.8 18.8 3 47003 III 34 M 18.8 18.8 18.8 18.8 4 15423 II 26 M 18.8 18.8 18.8 18.8 5 60245 III 44 F 18.8 21.6 18.8 18.8 6 66522 II 50 M 18.4 18.4 18.4 16.0 7 66400 III 45 M 16.0 21.2 21.2 21.2 8 47964 II 30 M 13.9 21.2 21.2 21.2 9 36939 II 29 F 16.0 16.0 21.2 18.4 10 57810 II 41 M 16.0 18.4 18.4 18.4 11 58977 III 39 M 16.0 18.4 18.4 18.4 12 67597 II 43 M 16.0 18.4 18.4 16.0 13 65072 III 33 F 16.0 16.0 16.0 16.0 14 64564 II 21 M 16.0 18.4 18.4 16.0 15 63032 III 36 F 16.0 16.0 16.0 16.0 16 64637 III 18 M 16.0 16.0 16.0 16.0 17 11735 II 49 M 18.4 21.2 21.2 21.2 18 7329 II 34 M 13.9 18.4 16.0 13.9 19 38010 II 29 M 16.0 18.4 18.4 16.0 20 65721 II 25 M 16.0 18.4 18.4 16.0 Average SPF (n = 20) 16.5 18.3 18.4 17.5 Standard deviation 1.62 2.21 1.96 2.20 Standard error 0.36 0.49 0.44 0.49 t (one-tail) 1.729 1.729 1.729 1.729 A 0.63 0.85 0.76 0.85 SPF label 15 17 17 16
JOURNAL OF COSMETIC SCIENCE 258 seconds, required to produce mild but defi nite erythema with clearly defi ned borders for the unprotected test site. More simply, MED protected site SPF MED unprotected site RESULTS The panel of subjects had 16 males (80%) and 4 females (20%), and had 13 Fitzpatrick skin phototype II (65%) and 7 Fitzpatrick skin phototype III (35%) subjects. There were no Fitzpatrick skin phototype I subjects. The subjects’ mean age was 36 years (minimum = 18 years maximum = 57 years). The SPF values obtained in this clinical trial are shown in Table I. The control standard exhibited an SPF of 16.5 (StDev = 1.62), which is consistent with the expected SPF of 16.3. The test site that had been treated with the control standard followed by sand #1961 exhibited an SPF of 18.3 (StDev = 2.2). The test site that had been treated with the con- trol standard followed by sand #1962 exhibited an SPF of 18.4 (StDev = 2.0). The test site that had been treated with the control standard followed by sand #1152 exhibited an SPF of 17.5 (StDev = 2.2). Thus, the SPF for each test site treated with the control standard followed by sand was essentially the same as the test site treated only with the control standard. There is no signifi cant difference in the average SPF with or without sand. CONCLUSIONS Under the conditions of this clinical trial, there is no signifi cant difference in the average SPF with or without sand. However, the fi ne sand was diffi cult to remove and the coarse sand was unpleasant to the subjects. Thus, our data suggests that Quickrete® commercial sand grade #1962 is preferred for sand-resistance testing. The next step should be the assessment of sand resistance in commonly used sunscreens, especially those with water-resistance claims. However, the Final Rule (3) does not allow for a sand-resistance SPF related claim, which suggests that additional research might fi rst require regulatory review. REFERENCES (1) Sunscreen drug products for over-the-counter human use, Final Monograph, Federal Register, 64, 27666–27693 (1999). (2) D. S. Berger, Specifi cation and design of solar ultraviolet simulators, J. Invest. Dermatol., 53, 192–199 (1969). (3) Labeling and effectiveness testing sunscreen drug products for over-the-counter human use, Federal Register, 76, 35620–35665 (2011).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)