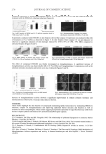
IN VITRO SPF DETERMINATION ON HD6 PMMA 245 STUDY OF AN AMPHOTERIC HD6 PRETREATMENT Surfactants properties. The aim of the present study was to control more reliably the inter- action between products and the HD6 substrate during spreading. In this regard, the properties of the surfactant were used in order to modify the molded PMMA plates on a more strongly hydrophilic surface. Indeed, surfactants are usually amphiphilic compounds (containing both hydrophobic and hydrophilic groups). Very few amounts of surfactant can modify the interfacial tension, particularly those of water, by adsorbing at the inter- faces thanks to their amphoteric nature. Interfaces are characterized by an interfacial level of energy that depends on the properties of the two separated phases (chemical composi- tion and the nature of both phases) (7–10). As a result of their properties, surfactants are able to increase surface wetability, which corresponds to the ability of a drop to spread on a solid surface (Figure 1). Wettability properties have great application in the areas of painting and surfaces (11,12), and the interfacial tensions between the different phases are related by the following equation: T J J J SL SV LV cos where JSV J J SL LV =interfacial tension of the interface solid gas =interfacial tension of the interface solid liquid =interfacial tension of the interface liquid gas The deposition of a drop of sunscreen on the HD6 offers different degrees of wettability according to the product’s nature: the more the contact angle formed on the PMMA Table I Sample A and B Description Products In vivo SPF Base Adherence product/substrate In vitro SPF on HD6 vs in vivo Sample A 16 Steareth-21, Steareth-2 Bad Poor correlation Sample B 30 Acrylate polymer Good Good correlation Figure 1. A. High interfacial tension. B. Low interfacial tension.
JOURNAL OF COSMETIC SCIENCE 246 increases, the more the cosine of this angle becomes small or even negative, and therefore the more the interfacial tension of the interface solid/liquid is important. Conversely, the more the contact angle decreases, the more the interfacial tension of the interface solid/ liquid also decreases. Wettability measurements. Wettability of untreated and pre-treated HD6 was tested by deionized water to evaluate the properties of the surface and the variation brought by the amphoteric treatment. Then the contact angles (θ) formed after the deposition of A and B on the HD6 with and without pretreatment were measured to compare the interfacial tension of both products according to the substrate properties. Measurements were performed by the Laboratory of Chemistry of Organic Materials and Metal, University of Nice-Sophia Antipolis. The contact angles (θ) were measured by a goniometer (Krüss DSA-10 contact angle goniometer). Drops of water and cream were deposited using a syringe controlled by a computer (fi xed volume) on the surface of the HD6 plate. The contact angle was determined from images captured by the computer via a camera (software drop shape analysis). SURFACTANT SELECTED FOR HD6 PRETREATMENT, COCAMIDOPROPYL BETAIN The amphoteric surfactant, cocamidopropyl betain (C.B.) (Figure 2) was selected as a pretreatment to decrease the hydrophobicity of the HD6 PMMA plates. (commercial name: TEGO® Betain F50). Supplied by Evonik Goldschmidt Industries, cocamidopropyl betain is more highly concentrated than common products incorporating 30% of raw material (13). IN VITRO SPECTROSCOPIC MEASUREMENTS ON HD6 AND PRETREATED HD6 All the spectroscopic data used in this study were based on transmission measurements of suncare products applied on HD6 with or without amphoteric pretreatment. The transmission spectrum for each area measured was determined, and then absolute protection factors like SPF were calculated by combining the UV transmission spectrum of the sun- screen preparation with a specifi c biological action spectrum and a relevant sun emission spectrum. Operating conditions. A Labsphere® UV-2000 S Transmittance analyzer was used in the deter- mination of the diffuse transmission spectrum of UV radiation through the substrate Figure 2. Chemical structure of cocamidopropyl betain. A. aliphatic tail. B. Polar head.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)