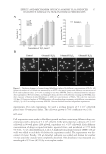
JOURNAL OF COSMETIC SCIENCE 2 acnes growth. The excessive lipids and improper differentiation of the keratinocytes in the follicle encourages bacterial growth and causes a weakening of the epidermal barrier in this follicle. This allows the bacteria and/or their metabolites to migrate into the skin and contribute to the infl ammation observed in the fi nal stages of the acne process (2–4). The fi nal phase of comedone formation is the infl amed lesion. The bacterial infi ltrate into the skin triggers infl ammatory mediator production and cellular infi ltrate. A variety of infl ammatory mediators have been described in the acne lesion. These include interleukin-1α (IL-1α), IL-1β, and substance P (5). In addition, there is a reported in- crease in lymphocytic infi ltrate and neutrophil infi ltrate in the follicular region (6), fur- ther contributing to the infl ammation associated with the acne lesion. This leads to further increased production of the proinfl ammatory cytokines, IL-1α and tumor necrosis factor-alpha by T cells and keratinocytes, leading to proliferation of both cell types (4). The primary pathogenic agent implicated in the development of infl ammatory and non- infl ammatory acne is P. acnes (7,8). P. acnes is included in a family of anaerobic, non-spore- forming gram-positive rods. The use of antibiotics to treat acne began in the 1960s however, in the last two decades several antibiotic-resistant strains have emerged (7–11). In addition to antibiotics, topical benzoyl peroxide (BP) (12,13) and salicylic acid have consistently been found to be effective in reducing acne lesions (14,15). Growing aware- ness of antibiotic-resistant Propionibacterium species has contributed toward increased use of topical and systemic differentiation agents like retinoid (16,17), which help reduce the hyperproliferation of keratinocytes and can inhibit the migration of leukocytes (1,6,9). Glucose oxidase catalyzes the oxidation of β-D-glucose to gluconic acid by utilizing mo- lecular oxygen as an electron acceptor with simultaneous production of hydrogen perox- ide (18). Such production of hydrogen peroxide from sources of glucose like honey has been shown to possess potent antibacterial properties (19) and has been used in wound healing (20,21). We have developed a hydrogen peroxide generating system using the enzyme glucose oxidase and glucose in an emulsion system that is stable and nonirritat- ing. This study was designed to evaluate the effect of this formulation system on reducing acne. METHODS AND MATERIALS IN VITRO CHALLENGE TEST An in vitro microbial challenge test was used to determine if the test material was effec- tive in eliminating microorganisms. Glucose oxidase (0.04%) and glucose substrate (0.36%), test material, were dissolved in tryptic soy broth (BD Difco, Sparks, MD) and challenged with fi ve pools of viable microorganisms known to contaminate cosmetics. Elimination of these microorganisms was followed over a 7-day period (22,23). Pool 1 Enterics contained Klebsiella pneumoniae (ATCC 1388), Escherichia coli (ATCC 8739), and Enterobacter gergoviae (ATCC 33028) Pool 2 Pseudomonas consisted of Burk- holderia cepacia (ATCC 25416), Pseudomonas stutzeri (ATCC 17588), Pseudomonas putida (ATCC 49128), and Pseudomonas aeruginosa (ATCC 9027) Pool 3 Staphylococci contained Staphylococcus aureus (ATCC 6538) and S. epidermidis (ATCC 49134) Pool 4 consisted of
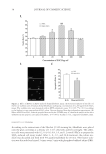
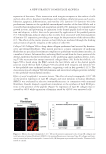
ENZYMATICALLY GENERATED HYDROGEN PEROXIDE REDUCES ACNE LESIONS 3 yeast, Candida albicans (ATCC 10231) and Pool 5 consisted of mold Aspergillus niger (ATCC 16404). The test material was prepared in sterile tryptic soy broth (BD Difco) and 20 g of each was transferred aseptically into fi ve sterile containers. Each 20-g sample was inoculated with 0.1 ml of appropriate inoculums such that each bacteria and yeast pool contained 106 colonies, whereas the mold pool contained 105 colonies. The container was tightly closed and the inoculated sample was mixed thoroughly and then incubated at ambient temperature (20–25°C). The samples were observed for viable microbes after 24 h, 48 h, and 7 days. At each time point, 1.0 ml of each well-mixed sample was transferred by a sterile pipette to test tubes containing 9-ml diluent (Trypticase Azolectin Tween Broth Base BD Difco) creating a 1:10 dilution of which 1.1 ml was dispensed into one sterile petri dish and 1.0 ml into another. Melted agar medium (15–20 ml) kept at 45–50°C was added to each petri dish and rotated to disperse the product and agar thoroughly. Tryptic soy agar (BD Difco) was used for inoculum Pools 1, 2, and 3 and potato dextrose agar (BD Difco) for inoculum Pools 4 and 5. Once the agar was solidifi ed, the petri dishes were inverted and incubated at 32–35°C for 48 h (23). After 48 h incubation, the petri dishes were examined for recovery of any inoculated organ- ism. The number of colonies was counted on the petri dish representing the 1:10 dilution, and multiplied by 10, and then converted to its appropriate log value. The number of colo- nies counted on the petri dish representing the 1:100 dilution was multiplied by 100, and then converted to its appropriate log value. When there were no colonies present on the 1:10 or 1:100 dilution petri dishes, the count was represented by the log value of 0.0 (23). CLINICAL Part I: short-term effect. Material A: 10% BP formulation in an oil-in-water emulsion. Ma- terial B: glucose oxidase enzyme 0.08%, glucose substrate 0.64% in an oil-in-water emulsion. Material C: glucose oxidase enzyme 0.5%, glucose substrate 4.0% in an oil- in-water emulsion. Since the effect of hydrogen peroxide is pH dependent, the formulations were buffered at 7.0. There were no added ions such as copper and iron in these formulations. The effect of the above materials was evaluated in a 1-week assay described previously (21). Ten women volunteers between the ages of 18 and 50 were recruited from a local population. All subjects were healthy with no evidence of acute or chronic disease other than acne. Written informed consent was obtained from each volunteer before entrance into the study. The panelists were not on any antibiotic, antihistamines, retinoid, anti- infl ammatories or steroid therapy, and BP and/or salicylic acid treatment for at least 2 weeks before commencement of this study. The subjects were not under the care of a der- matologist and were not on any acne treatment for at least 1 month before the study started. Pregnant or lactating women were excluded. The panelists exhibited acne with at least two closed comedones on the upper back, minimum distances between closed comedones were approximately 4–6 cm. Two infl amed acne lesions were selected for each treatment and one for the untreated. Each lesion was marked, photographed, and graded. A skin surface microscope (Scopeman
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)