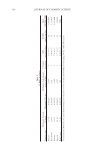
Table VI Co MoS Calculation Cosmetic type Maximum detected Co (μg/g) Maximum %Co (g) Estimated daily amount applied (g/day) % Dermal absorption SED (mg/kg bw/day) NOAEL (mg/kg bw/day) MoS (NOAEL/SED) Mascara 10.18 0.001018 0.025 0.13 5.51 × 10−8 0.01 181,351 Eye shadow 7.09 0.000709 0.02 0.13 3.07 × 10−8 0.01 325,486 Eyeliner 48.19 0.004819 0.005 0.13 5.22 × 10−8 0.01 191,549 Lipstick 1.44 0.000144 0.057 0.13 1.78 × 10−8 0.01 562,303 Body lotion 0.81 0.000081 7.82 0.13 8.23 × 10−5 0.01 7286 According to our results, all the MoS values for the metals tested were found to be higher than 100 that is accepted as a minimum value for safe use of a substance (19). ALLERGEN METALS IN COSMETICS 321
JOURNAL OF COSMETIC SCIENCE 322 It should be highlighted that in the fi rst step of hazard identifi cation, determination of the intrinsic factors that may produce potential risk to human health should be consid- ered (1). Therefore, although removal of heavy metals from personal care products after manufacturing is not possible, careful selection of the raw material can improve the qual- ity of the products (15). Moreover, postmarketing vigilance is very important to protect public health, although the regulation on cosmetovigilance system is handled differently in many countries (27). Finally, it must be emphasized that contact dermatitis caused by cosmetics may be due to metal content therefore, to assess the safety of the fi nished products, routine monitoring of allergen metal content is crucial. REFERENCES (1) V. Rogiers and M. Pauwels, “Safety Assessment of Cosmetics in Europe,” in Current Problems in Dermatol- ogy, P. Itin. Ed. (Karger, Basel, Switzerland, 2008), Vol. 36, pp. 1–28. (2) P. Engasser, T. Long, P. McNamee, H. Schlatter, and J. Gray, Safety of cosmetic products, J. Cosmet. Dermatol., 6, 23–31 (2007). (3) P. K. Nigam, Adverse reactions to cosmetics and methods of testing, Indian J. Dermatol. Venereol. Leprol., 75, 10–18 (2009). (4) D. A. Basketter, G. Angelini, A. Ingber, P. S. Kern, and T. Menné, Nickel, chromium, and cobalt in consumer products: Revisiting safe levels in the new millennium, Contact Dermatitis, 49, 1–7 (2003). (5) I. Duarte, J. R. Amorim, E. Félix, and R. S. Junior, Metal contact dermatitis: Prevalence of sensitization to nickel, cobalt, and chromium, An Bras Dermatol., 80, 137–142 (2005). (6) N. Fyhrquist, E. Lehto, and A. Lauerma, New fi ndings in allergic contact dermatitis, Curr. Opin. Allergy Clin. Immunol., 14, 430–520 (2014). (7) Council Directive 76/768/EEC, On the Approximation of the Laws of the Member States Relating to Cosmetic Products (That Cosmetic Must Not Contain Cd and Cr), 1976, accessed March 30, 2015, http://eurlex. europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31976L0768:EN:html (8) Cosmetic Legislation, TC SağlıkBakanlığı, Kozmetik Yönetmeliği, 2006, accessed March 30, 2015, http:// www.saglik.gov.tr/TR/belge/1-472/kozmetik-yonetmeligi.html (9) FDA, Summary of Color Additives Listed for Use in the United States in Food, Drugs, Cosmetics and Medical Devices, Color Additives Approved for Use in Cosmetics Part 73, Subpart C: Color Additives Exempt From Batch Certification, 2007, accessed March 30, 2015, http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&SID= fc2869830da702969a2dcfdeb71d7594&rgn=div5&view=text&node=21:1.0.1.1.27&idno=21#sp21.1.73.c (10) R. Wolf, E. Orion, and Y. Tüzün, Periorbital (eyelid) dermatides, Clin. Dermatol., 32, 131–140 (2014). (11) K. A. Zug, R. Kornik, D. V. Belsito, V. A. DeLeo, J. F. Fowler, H. I. Maibach, J. G. Marks, C. G. Mathias, M. D. Pratt, R. L. Rietschel, D. Sasseville, F. J. Storrs, J. S. Taylor, and E. M. Warshaw, North American Contact Dermatitis Group, Patch-testing North American lip dermatitis patients: Data from the North American Contact Dermatitis Group, 2001 to 2004, Dermatitis, 19, 202–208 (2008). (12) H. Sipahi, M. Charehsaz, I. Sonmez, B. Soykut, O. Erdem, and A. Aydin, Assessment of cadmium, lead, and nickel levels in hair care products marketed in Turkey, J. Cosmet. Sci., 65, 239–244 (2014). (13) J. P. Thyssen, J. D. Johansen, A. Linneberg, and T. Menné, The epidemiology of hand eczema in the general population—Prevalence and main fi ndings, Contact Dermatitis, 62, 75–87 (2010). (14) R. I. Nijhawan and S. E. Jacob, Contact alternatives to nickel, Dermatitis, 24, 222–226 (2013). (15) B. Bocca, A. Pino, A. Alimonti, and G. Forte, Toxic metals contained in cosmetics: A status report, Regul. Toxicol. Pharmacol., 68, 447–467 (2014). (16) D. Basketter, L. Horev, D. Slodovnik, S. Merimes, A. Trattner, and A. Ingber, Investigation of the threshold for allergic reactivity to chromium, Contact Dermatitis, 44, 70–74 (2001). (17) H. Ullah, S. Noreena, Fozia, A. Rehman, A. Waseem, S. Zubair, M. Adnan, and I. Ahmad, Com- parative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan, Arabian J. Chem., In Press, available online Sept. 20, 2013. doi:10.1016/ j.arabjc.2013.09.021.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)