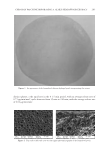
J. Cosmet. Sci., 68, 245–252 ( July/August 2017) 245 Assessment of bacterial contamination of lipstick using pyrosequencing SO Y. LEE and SI Y. LEE Department of Oral Microbiology, Research Institute of Oral Science, Gangneung-Wonju National University, Gangneung, 210-702, Korea Acceptance of publication May 7, 2017. Synopsis As soon as they are exposed to the environment, cosmetics become contaminated with microorganisms, and this contamination accumulates with increased use. In this study, we employed pyrosequencing to investigate the diversity of bacteria found on lipstick. Bacterial DNA was extracted from 20 lipstick samples and mixed in equal ratios for pyrosequencing analysis. As a result, 105 bacterial genera were detected, four of which (Leifsonia, Methylobacterium, Streptococcus, and Haemophilus) were predominant in 92% of the 19,863 total sequence reads. Potentially pathogenic genera such as Staphylococcus, Pseudomonas, Escherichia, Salmonella, Corynebacterium, Mycobacterium, and Neisseria accounted for 27.6% of the 105 genera. The most commonly identifi ed oral bacteria belonged to the Streptococcus genus, although other oral genera such as Actinomyces, Fusobacterium, Porphyromonas, and Lactobacillus were also detected. INTRODUCTION Cosmetics should be free from pathogenic microorganisms while also maintaining a low count of aerobic microorganisms (1). Microorganism-contaminated cosmetics can affect product quality and pose serious consumer health risks if the contaminating agents are pathogenic (1,2). In general, bacteria such as Staphylococcus aureus, Escherichia coli, Salmonella spp., Candida albicans, Clostridium spp., and Pseudomonas aeruginosa should be absent (3). Lipstick is at risk of contamination by airborne microorganisms and saliva from the moment it is opened and contacts the lips and skin, a risk that only increases with use (4). Although diverse bacterial species have been isolated from lipstick, most studies to date have been limited to using counts from culturable aerobic bacteria (3–6). In recent years, however, it has become possible to detect the presence of unculturable bacteria using pyrosequencing. In this study, we used pyrosequencing to examine the diversity of con- taminating bacteria in 20 lipstick samples. Address all correspondence to Si Young Lee at siyoung@gwnu.ac.kr.
JOURNAL OF COSMETIC SCIENCE 246 MATERIALS AND METHODS SAMPLING Twenty lipstick samples used by women between the ages of 20 and 50 were tested for microbial contamination. Before opening the sample cap, the lipstick surface was cleaned with 70% ethanol. The surface of the lipstick was then swabbed using a sterile cotton swab. Samples of the swab surface were suspended in 1 ml of sterile distilled water. GENOMIC DNA EXTRACTION For extraction of bacterial DNA from each of the 20 lipstick samples, G-spin Genomic DNA Extraction Kit for bacteria (Intron Biotechnology Inc., Seongnam-si, Korea) was used, and DNA was extracted according to the manufacturer’s instructions. Pyrosequencing analysis was performed once with the target sample consisting of the 20 DNA samples mixed in equal ratios. PYROSEQUENCING Polymerase chain reaction (PCR) amplifi cation was performed using primers targeting the V3 to V4 regions of the 16S rRNA gene found in the extracted DNA. For bacterial amplifi cation, primers 341F (5′-TCGTCGGCAGCGTC-AGATGTGTATAAGAGACAG- CCTACGGGNGGCWGCAG-3′ underlining sequence indicates the complimentary region of the primer) and 805R (5′-GTCTCGTGGGCTCGG-AGATGTGTATAAGAGACAG- GACTACHVGGGTATCTAATCC-3′) were used. The amplifi cations were carried out under the following conditions: initial denaturation at 95°C for 3 min, followed by 25 cycles of denaturation at 95°C for 30 s, primer annealing at 55°C for 30 s, and extension at 72°C for 30 s, with a fi nal elongation at 72°C for 5 min. Secondary amplifi cation for attaching the Illumina NexTera barcode was performed with i5 forward primer (5′-AAT- GATACGGCGACCACCGAGATCTACAC-XXXXXXXX-TCGTCGGCAGCGTC-3′ X indicates the barcode region) and i7 reverse primer (5′-CAAGCAGAAGACGGCATAC- GAGAT-XXXXXXXX-AGTCTCGTGGGCTCGG-3′). Conditions for secondary amplifi cation were similar to the previous one except with eight cycles of amplifi cation. The correct PCR product was confi rmed using electrophoresis on a 2% agarose gel followed by visualization under a Gel Doc system (BioRad, Hercules, CA). The amplifi ed products were purifi ed using the QIAquick PCR purifi cation kit (Qiagen, Valencia, CA). Equal concentrations of purifi ed products were pooled together and short fragments corresponding to nontarget products were removed with the Ampure bead kit (Agencourt Bioscience, Beverly, MA). Sample quality and product size were assessed using a Bioanalyzer 2100 (Agilent, Palo Alto, CA) and a DNA 7500 chip (Agilent). Mixed amplicons were pooled and the sequencing was carried out at Chunlab, Inc. (Seoul, Korea) using an Illumina MiSeq Sequencing system (Illumina, San Diego, CA) according to the manufacturer’s instructions. SEQUENCING DATA ANALYSIS Obtained reads were sorted using the unique barcodes of each PCR product. The barcode, linker, and primer sequences were removed from the original reads. Any reads containing
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)