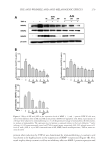
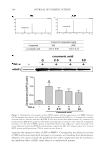
CHITOSAN PATCH INCORPORATING A. ALTILIS HEARTWOOD EXTRACT 259 at 282 nm using a calibration curve of a standard artocarpin. The study was performed in triplicates. The standard artocarpin used in this study was isolated from a diethyl ether extract of A. altilis heartwood, according to previous studies (7,11). FORMULATION OF CHITOSAN HYDROGEL PATCH INCORPORATING THE EXTRACT Preparation of the extract into the oil-in-water (o/w) microemulsion. The o/w microemulsion system consisted of 0.04% w/w extract powder, 1% w/w isopropyl myristate (IPM Nikko Chemicals Pte. Ltd., Jurong Island, Singapore), 12.8% w/w polyoxyethylene sorbitan monosterate (Tween® 80 Nof Corporation, Tokyo, Japan), 6.4% w/w glycerin (Namsiang Trading Co., Ltd., Bangkok, Thailand), and 79.4% w/w of deionized (DI) water. The extract powder was dissolved in IPM to obtain the internal oil phase of the microemulsion sys- tem. The external water phase consisted of Tween® 80, glycerin, and DI water. The water phase was continuously added to the oil phase with slightly mixing. The obtained micro- emulsion was transparent, and the mean hydrodynamic diameter of its internal oil phase was 31.8 ± 1.2 nm as measured thrice by photon correlation spectroscopy employing a Zetasizer (Model ZetaPALS Brookhaven Instruments Coporation, Holtsville, NY). Preparation of chitosan solution. The chitosan used had a molecular weight in the range of 100,000–1,000,000 Dalton and more than 95% degree of deacetylation (Aqua Premier Co., Ltd, Chonburi, Thailand). The total heavy metal and ash contents in chitosan were less than 10 ppm and 2%, respectively. Chitosan with a specifi ed amount at 4% w/w was dispersed in a part of DI water. Then, lactic acid (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was added to dissolve the chitosan and to control the pH of the chitosan solution in a range of 3–4. The chitosan–lactic acid mixture was stirred at room temperature until a clear yellowish solution was obtained. Formulation of a chitosan hydrogel patch incorporating the extract. The o/w microemulsion containing 0.04% w/w extract was blended with the 4% w/w chitosan solution in a ratio of 1 to 1 by weight. NaCl (Ajax Finechem Pty. Ltd., New South Wales, Australia) at an amount of 1% w/w was then added in the blended mixture. The blended mixture was further agitated for 1 h and left standing until free from air bubbles. The 21 grams of the resultant solution was cast on a clear petri dish with a diameter of 9 cm and kept on a level surface at 35°–40°C in a hot-air oven. After 2–3 d, the casted patch was peeled off from the petri dish and kept for further determinations. The amount of artocarpin in the patch was 0.07 mg/cm2, according to analysis by using HPLC. The thickness of an indi- vidual patch was controlled to 500 ±10 μm. DETERMINATION OF PHYSICOCHEMICAL PROPERTIES OF THE FORMULATED PATCH INCORPORATING THE EXTRACT Mechanical properties. The tensile tester (Instron® Model 4411 S/N H2082 Instron Ltd., Buckinghamshire, UK) was used to determine the tensile strength and percent elonga- tion at break of the formulated patches. The patch specimens were cut out in a rectangle with a length of 70.0 mm and a width of 10.0 mm. The thickness of each specimen was calculated as the average value of three separate measurements taken along the middle of 20 mm. The cross-section area of the tested patch was calculated by multiplying the mean thickness with gauge width. The tested patch was clamped by upper and lower grips. The rate of grip separation was 12.5 mm/min and loading weight was 200 N. The testing
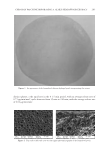
JOURNAL OF COSMETIC SCIENCE 260 room condition was controlled at 23 ± 2°C with 40–70% relative humidity. The ultimate tensile strength and percent elongation at break value were calculated by these formulas: Ultimatetensilestrength(kg/mm2) breakingload cross-sectionareaof thetestedspecimen Percentelongationatbreak Differentinthelengthatbreakingpoint 100 originallengthof thetestedspecimen = = × MOISTURE CONTENT Moisture analyzer (Sartorius Model MA30, DKSH GmbH, Hamburg, Germany) was used to measure the percentage of moisture content of the formulated patches. The tested patches were cut in 0.2 × 0.2 cm2 and approximately 5 g was placed in a preweighted aluminum dish. The dishes and contents were put in an oven at 105°C for 12 min and then placed in a desiccator to cool down before weighing. The percentage of moisture content was calculated from the below formula: % Moisturecontent Initialweight Finalweight 100 Initial weight = - × The experiment was performed in triplicate. Surface morphology. The surface morphology of the formulated patch was determined by using a scanning electron microscope (SEM Model 1455VP LEO, Cambridge, UK). The tested patches were dried in a hot-air oven at 40°C and kept in a desiccator to constant weight before testing. Double-sided tape was used to keep the dried patch attached on the stub. All tested patches on stub were coated with gold. All images were manifested at 350 times. IN VITRO RELEASE STUDY OF ARTOCARPIN FROM THE FORMULATED PATCH INCORPORATING THE EXTRACT In vitro release of artocarpin from the formulated patches was evaluated by using the vertical Franz diffusion cell (Model V6A-02/90824 PermeGear Inc., Hellertown, PA). The area of the tested patch exposed to the phosphate buffer (pH 7.4), the receptor medium, was 2.27 cm2. The volume of the phosphate buffer in the receptor chamber was 12 mL with the temperature set at 37°C. This whole assembly was kept on a magnetic stirrer, and the receptor medium was stirred continuously using a magnetic bead. The receptor medium were collected at various time intervals (5, 10, 15, 30, 60,120, and 240 min) and replaced with an equal volume of fresh medium. The amount of artocarpin that diffused through the patch and accumulated in the receptor medium was determined by HPLC. The study was run in triplicate. EFFICACY AND TOLERANCE STUDY OF THE FORMULATED PATCH INCORPORATING THE EXTRACT The study protocol was approved by Human Ethical Committee, Naresuan University, Phitsanulok, Thailand with the permission number 54 03 03 0004. All procedures were
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)