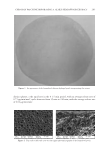
BACTERIAL CONTAMINATION OF LIPSTICK 251 after use (4). Proteus, Providencia, Morganella, Staphylococcus, and Pseudomonas were detected using 16S rDNA sequencing, Gram stain, and biochemical characterization. Although these studies are limited to culturable bacteria, they have nonetheless detected the pres- ence of various genera of bacteria on lipstick. Preservatives are commonly used to ensure the stability and safety of cosmetic products (14,17). Various preservatives have been used to maintain low levels of microorganism contamination and to increase the shelf life of lipstick (4,18). Air contact following open- ing of the cap has been reported to increase microbial contamination, although preserva- tives possess suffi cient antimicrobial activity to maintain product safety (4). In our study, 20 lipstick samples were plated on a blood agar plate and cultured in a 5% CO2 incubator for 48 h. Live bacteria were found in seven of 20 samples (data not shown). These results suggest that the antiseptics contained in lipstick may be present in insuffi cient concen- trations for antimicrobial activity resulting in contamination. In this study, we investigated the diversity of contaminating bacteria in lipstick using pyrosequencing. We detected a wider diversity of contaminating bacteria compared with previous studies. Lipstick comes into direct contact with the mouth, and because it is reused, microorganisms can infect the skin as well. We suggest that consumers should use products that inhibit the growth of contaminating bacteria on their cosmetics, and that the types of preservatives as well as their concentrations should be optimized. REFERENCES (1) J. Behra van, F. Bazzaz, and P. Malaekeh, Survey of bacteriological contamination of cosmetic creams in Iran (2000). Int. J. Dermatol., 44, 482–485 (2005). (2) R. Campa na, C. Scesa, V. Patrone, E. Vittoria, and W. Baffone, Microbiological study of cosmetic products during their use by consumers: Health risk and effi cacy of preservative systems. Lett. Appl. Microbiol., 43, 301–306 (2006). Figure 1. Dive rsity of the bacterial community present in lipstick samples.
JOURNAL OF COSMETIC SCIENCE 252 (3) K. O. Fa tma, Ö. Selda, and A. Duygu, Microbiological investigation of used cosmetic samples. Hacettepe Univ. J. Faculty Pharm., 30, 1–16 (2010). (4) S. S. Sne ha and K. M. Varsha, Study of bacterial contaminants in local as well as branded lipsticks before and after consumer use. Int. J. Recent Adv. Multidiscip. Res., 2, 149–154 (2015). (5) J. Basil, J. R. Alan, C. R. Annette, and A. Michael, A survey of microbiological contamination in cos- metics and toiletriesin the U.K. (1971). J. Soc. Cosmet Chem., 25, 563–575 (1974). (6) H. J. Muh ammed, Bacterial and fungal contamination in three brands of cosmetics marketed in Iraq. Iraqi J. Pharm. Sci., 20, 38–42 (2011). (7) T. Huber, G. Faulkner, and P. Hugenholtz, Bellerophon: A program to detect chimeric sequences in multiple sequence alignments. Bioinformatics, 20, 2317–2319 (2004). (8) O. S. Kim , Y. J. Cho, K. Lee, S. H. Yoon, M. Kim, and H. Na, Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol., 62, 716–721 (2012). (9) L. Han, J . E. Lei, X. Wang, L. T. Guo, Q. Y. Kang, and L. He. Septicemia caused by Leifsonia aquatica in a healthy patient after retinal reattachment surgery. J. Clin. Microbiol., 51, 3886–3888 (2013). (10) L. Porte , A. Soto, D. Andrighetti, J. Dabanch, S. Braun, and A. Saldivia, Catheter-associated blood- stream infection caused by Leifsonia aquatica in a haemodialysis patient: A case report. J. Med. Microbiol., 61, 868–873 (2012). (11) A. Zinch uk, O. Zubach, A. Zadorozhnyj, Y. Chudina, and V. Bilavka, Characteristics of meningitis due to Methylobacterium mesophilicum: A rare case. Jpn. J. Infect Dis., 68, 343–346 (2015). (12) H. F. Je nkinson, H. C. Lala, and M. G. Shepherd, Coaggregation of Streptococcus sanguis and other strep- tococci with Candida albicans. Infect Immun., 58, 1429–1436 (1990). (13) N. Norsk ov-Lauritsen, Classifi cation, identifi cation, and clinical signifi cance of Haemophilus and Aggre- gatibacter species with host specifi city for humans. Clin. Microbiol. Rev., 27, 214–240 (2014). (14) F. Devli eghere, A. De Loy-Hendrickx, M. Rademaker, P. Pipelers, A. Crozier, and B De Baets, A new protocol for evaluating the effi cacy of some dispensing systems of a packaging in the microbial protec- tion of water-based preservative-free cosmetic products. Int. J. Cosmet. Sci., 37, 627–635 (2015). (15) T. L. Ha dfi eld, P. McEvoy, Y. Polotsky, V. A. Tzinserling, and A. A. Yakovlev, The pathology of diph- theria. J. Infect. Dis., 181, S116–S120 (2000). (16) L. Plant , V. Asp, L. Lovkvist, J. Sundqvist, and A. B. Jonsson, Epithelial cell responses induced upon adherence of pathogenic Neisseria. Cell Microbiol., 6, 663–670 (2004). (17) M. D. Lu ndov, L. Moesby, C. Zachariae, and J. D. Johansen, Contamination versus preservation of cos- metics: A review on legislation, usage, infections, and contact allergy. Contact Dermatitis., 60, 70–78 (2009). (18) K. Rajgo pal and S. S. Agarwal, Simultaneous estimation of p-hydroxybenzoic acid and its esters in wash off/leave- on cosmetic products by highperformance thin layer chromatography. Int. J. Pharm. Stud. Res., 2, 100–105 (2011).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)