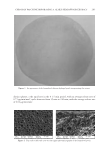
NON-COMEDOGENIC AND NON-ACNEGENIC CLAIM SUBSTANTIATION 255 Subjects must have not more than 25 facial lesions, which are predominately open and closed comedones. A board-certifi ed dermatologist evaluates the lesions on the face and the results are recorded. Typically, the subject uses the test material on the face for 4–6 weeks. For a 4-week trial, evaluations occur after 2 weeks (optional) and 4 weeks of test material use, whereas for a 6-week trial, evaluations occur after 3 weeks (optional) and 6 weeks of test material use. A board-certifi ed dermatologist evaluates the lesions on the face and the results are recorded. Differences between baseline and interim or fi nal evaluations are considered statistically signifi cant if the probability of obtaining the results by chance is 0.050 using analysis of variance and/or t-test statistical analysis. BENEFITS The in-use clinical trial is a better clinical test than the follicular biopsy and a more pre- dictive test than the follicular biopsy. Like the follicular biopsy model, the in-use clinical trial is human based. However, the in-use clinical trial is the gold standard for comedogenicity because it uses the test material in the manner expected and, it uses the skin of the face, which is more susceptible to comedones and acne. In this regard, the in-use clinical trial is similar to acne trials used to support approval of acne medications by the Food and Drug Administration. A positive control, a material known to cause comedones, and a negative control, a material known not to cause comedones, are not needed because each subject at baseline serves as their own control. Each subject can generate comedones because they must have comedones to qualify for the trial. Thus, each subject enters the trial with the capacity to generate comedones. Consequently, a positive control is not necessary. Indeed, one could argue that including a positive control would be in confl ict with the principles of Good Clinical Practice as described in the World Medical Association’s Declaration of Helsinki (as amended). Other examples of a clinical trial in which a positive control is unethical and inhuman are allergy trials, such as photoallergy. In conclusion, the in-use clinical trial is a better model than the follicular biopsy trial, based on facial skin instead of back skin, and based on the use of the test material in a manner intended. The in-use clinical trial is the gold standard. RISKS The primary risk of the in-use clinical trial is the expense. Conducting an in-use clinical trial is more expensive than the follicular biopsy model, which is why some companies do not conduct in-use clinical trials. CONCLUSIONS In conclusion, the in-use clinical trial is the gold standard when determining comedo- genicity of an ingredient or product. The follicular biopsy model has defi ciencies com- pared with the in-use clinical trial, and the rabbit model has defi ciencies compared with the follicular biopsy model. Although more expensive, the in-use clinical trial uses the face,
JOURNAL OF COSMETIC SCIENCE 256 which is most susceptible to comedones and acne and uses the test material in an expected manner. A positive and negative control is not required because each subject serves as its own control. Statistical analysis compares pretreatment baseline lesion counts with post- treatment lesion counts. REFERENCES (1) A. M. Kligman and O. H. Mills, Acne cosmetic, Arch. Dermatol., 106, 893–897 (1972). (2) A. M. Kligman and T. Kwong, An improved rabbit ear model for assessing comedogenic substances, Br. J. Dermatol., 100, 699–702 (1979). (3) W. E. Morris and S. C. Kwan, Use of the rabbit ear model in evaluating the comedogenic potential of cosmetic ingredients, J. Soc. Cosmet. Chem., 34, 215–225 (1983). (4) J. E. Fulton, Comedogenicity and irritancy of commonly used ingredients in skin care products, J. Soc. Cosmet. Chem., 40, 321–333 (1989). (5) J.E. Fulton Jr, S. R. Pay, and J. E. Fulton 3rd, Comedogenicity of current therapeutic products, cosmet- ics, and ingredients in the rabbit ear, J. Am. Acad. Dermatol., 10, 96–105 (1984). (6) O. H. Mills and A. M. Kligman, Human model for assessing comedogenic substances, Arch. Dermatol., 118, 903–905 (1982). (7) P. G. Engasser, A. M. Kligman, P. Lazar, J. J. Leyden, P. E. Pochi, A. R. Shalita, and J. S. Strauss, Consensus statement. American Academy of Dermatology Invitational Symposium on Comedogenicity. J. Amer. Acad. Dermatol., 20, 272–277 (1989). (8) Z. D. Draelos and J. C. DiNardo, A re-evaluation of the comedogenicity concept, J. Am. Acad. Dermatol., 54, 507–512 (2006).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)