J. Cosmet. Sci., 68, 253– 256 ( July/August 2017) 253 Non-comedogenic and non-acnegenic claim substantiation CRAIG WEISS and MICHAEL CASWELL Consumer Product Testing Company, Inc., Fairfi eld, NJ 07004 Accepted for publication May 20, 2017. Synopsis There are currently two methods to evaluate comedogenecity. One is the inexpensive human model developed by Mills and Kligman and modifi ed by others. The second is the more costly human clinical trial, which is the gold standard for comedogenesis and to which the human model is compared. The qualifi cation of each method to support the comedogenecity claim is evaluated and contrasted. BACKGROUND “Acne cosmetic” was a term created by Mills and Kligman (1) to describe the development of comedones and/or acne in patients, typically middle-aged females, who would not normally be expected to develop such. The rabbit ear model was quickly developed and large amounts of data were generated using the model (2–5). The rabbit model was not a perfect predictor of comedogenesis in a human model (5,6). In 1989, at an invitational symposium on comedogenicity, the group wrote “If the animal model does not show evidence of comedogenesis, the test material under consideration is unlikely to be comedogenic in human skin (7).” Thus, the experts in 1989 wrote that the rabbit model did not accurately mimic comedogenesis in humans. Whereas the rabbit model is an adequate fi rst screen for comedogenicity, its inherent inability to mimic human comedogenesis has relegated it to a screening tool. There are currently two methods to evaluate comedogenecity. One is the inexpensive human model developed by Mills and Kligman and modifi ed by others (8). The second is the more costly human clinical trial, which is the gold standard for comedogenesis and to which the human model is compared. FOLLICULAR BIOPSY MODEL METHOD Individuals with prominent follicles on the upper back are recruited into the clinical trial. The upper back is patched with approximately 0.2 ml of each test material for 48 h Address all correspondence to Michael Caswell at MCaswell@cptclabs.com.
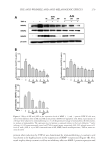
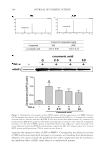
JOURNAL OF COSMETIC SCIENCE 254 (M, W) or 72 h (F), ensuring continuous exposure to the test material over 4 weeks. A positive and negative control must be included because of the wide variation in responses (7). A follicular biopsy is taken of the test sites. Cyanoacrylate glue is applied to a glass slide, which is then turned upside down and placed fi rmly on the skin at the test site, so that the glue is in contact with the test site. Upon drying, the slide is rapidly ripped off the skin to maintain the integrity of the biopsy specimen. The slide is then examined micro- scopically for the number of follicles and microcomedones per area. Some calculate the ratio of follicles to microcomedones to calculate the percent microcomedone formation. BENEFITS For more than 30 years, this human model of comedogenesis has been used as a tool by dermatologists to evaluate the ability of a cosmetic ingredient/product to induce comedo- nes. Follicular biopsy is a better model system than the rabbit model. Unlike the rabbit model, the follicular biopsy model is human based, increasing the expected relevance of the generated data. The follicular biopsy method seems to be more predictive than the rabbit model. Although the follicular biopsy model system is human based, the model system is relatively inexpensive. In conclusion, the follicular biopsy model system is a better model than the rabbit system, human based, and relatively inexpensive. RISKS Despite more than 30 years of use, the follicular biopsy model system has never been vali- dated against an in-use clinical trial, the gold standard. Thus, the relevance of the data generated by the follicular biopsy method should not be used to claim non-comedogenecity of an ingredient or fi nished product. Furthermore, the data generated by the follicular biopsy method should not be used to claim comedogenicity of a fi nished product, because the model system does not predict that fi nished products containing comedogenic ingredients are comedogenic (7). One reason for the questionable relevance of the follicular biopsy model is that most cos- metic products are used on the face or facial area, not on the back. The relationship of comedone/acne formation on the back to formation on the face has never been established. Although follicular biopsy has been performed on the face, this practice is not encouraged because of the potential for scarring when the glass slide is rapidly ripped from the skin. This is also a concern on the back biopsies, but of less concern to most subjects. In conclusion, the follicular biopsy model system has never been validated against the gold standard, comedones developed on the back have never been validated as a model for the face, the test material is used in a manner not intended, and the follicular biopsy model can induce scarring on subjects. IN-USE CLINICAL TRIAL METHOD Individuals with comedones and/or acne are recruited into the clinical trial. Subjects must have Grade I (mild), Grade II (moderate), or Grade III (severe) lesion categories.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)