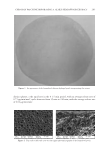
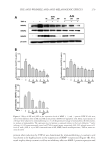
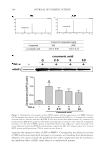
CHITOSAN PATCH INCORPORATING A. ALTILIS HEARTWOOD EXTRACT 269 (2) K . Shimizu, R. Kondo, K. Sakai, S. H. Lee, and H. Sato, The inhibitory components from Artocarpus incisus on melanin biosynthesis, Planta Med., 64, 408–412 (1998). (3) K . Shimizu, R. Kondo, K. Sakai, N. Takeda, and T. Nagahata, The skin-lightening effects of artocarpin on UVB-induced pigmentation, Planta Med, 68, 79–81 (2002). (4) S . Buranajaree, P. Donsing, R. Jeenapongsa, and J. VIyoch, Depigmenting action of a nanoemulsions containing heartwood extract of Artocarpus incisus on UVB-induced hyperpigmentation in C57BL/6 mice, J. Cosmet. Sci., 62, 1–14 (2011). (5) P . Donsing, N. Limpeanchob, and J. Viyoch, Evaluation of the effect of Thai breadfruit’s heartwood extract on melanogenesis-inhibitory and antioxidation activities, J. Cosmet. Sci., 59, 41–58, (2008). (6) N . Tiraravesit, S. Yakaew, R. Rukchy, W. Luangbudnark, C. Viennet, P. Humbert, and J. Viyoch, Arto- carpus altilis heartwood extract protects skin against UVB in vitro and in vivo, J. Ethnopharmacol., 175, 153–162 (2015). (7) K . Itsarasook, K. Ingkaninan, and J. Viyoch, Artocarpin-enriched extract reverse collagen metabolism in UV-exposed fi broblasts, Biologia, 69, 943–951 (2014). (8) W . C. Lan, C. W. Tzeng, and C. C. Lin, Prenylated fl avonoids from Artocarpus altilis: Antioxidant ac- tivities and inhibitory effects on melanin production, Phytochemistry, 89, 78–88 (2013). (9) P . Boriwanwattanarak, K. Ingkaninan, N. Khorana, and J. Viyoch, Development of hydrogel patch for controlling the release of curcuminoids by using chitosan as controlled-release matrix, Int. J. Cosmet. Sci., 30, 205–218 (2008). (10) J. Viyoch, T. Sudemek, W. Srema, and W. Suwongkrua, Development of hydrogel patch for controlled delivery of alpha-hydroxy acid contained in the extract of tamarind’s fruit pulp, Int. J. Cosmet. Sci., 27, 89–99 (2005). (11) T. Pitaksuteepong, A. Somsiri, and N. Waranuch, Targeted transfollicular delivery of artocarpin extract from Artocarpus incisus by means of microparticles, Eur. J. Pharm. Biopharm., 67, 639–645 (2007). (12) J. Viyoch, Mechanisms of Action of Artocarpus incisus’s Heartwood Extract on Melanogenesis Inhibi- tion, Final Report, Bangkok, Thailand: Thailand Research Fund, Contract No.: DBG5180012 (2009). (13) J. Viyoch, P. Patcharaworakulchai, R. Songmek, V. Pimsan, and S. Wittaya-Areekul, Formulation and development of a patch containing tamarind fruit extract by using the blended chitosan-starch as a rate- controlling matrix, Int. J. Cosmet. Sci., 25, 113–125 (2003). (14) N. Mondal, A. Samanta, T. K. Pal, and S. K. Ghosal, Effect of different formulation variables on some particle characteristics of poly (DL-lactide-co-glycolide) nanoparticles, Yakugaku Zasshi, 128, 595–601 (2008). (15) Q. Meng, M. C. Heuzey, and P. J. Carreau, Hierarchical structure and physicochemical properties of plasticized chitosan, Biomacromolecules, 15, 1216–1224 (2014). (16) B. Zhang, D. A. Hoagland, and Z. Su, Ionic liquids as plasticizers for polyelectrolyte complexes, J. Phys. Chem B, 119, 3603–3607 (2015). (17) L. Moreau, W. Bindzus, and S. Hill, Infl uence of sodium chloride on color development of cereal model systems through changes in glass transition temperature and water retention, Cereal Chem., 86, 232–238 (2009). (18) T. Karbowiak, H. Hervert, L. Léger, D. Champion, F. Debeaufort, A. Voilley, Effect of plasticizers (wa- ter and glycerol) on the diffusion of a small molecule in iota-carrageenan biopolymer fi lms for edible coating application, Biomacromolecules, 7, 2011–2019 (2006). (19) V . Epure, M. Griffon, E. Pollet, and L. Avétous, Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading, Carbohydr Polym, 83, 947–952 (2011). (20) M. F. Cardinal, M. Lovino, and D. Bernik, Comparative study of the porosity induced by CTAB and Tween as silica templates, Mater Sci Eng C, 27, 75–79 (2007). (21) S. Honary, B. Hoseinzadeh, and P. Shalchian, The effect of polymer molecular weight on citrate cross- linked chitosan fi lms for site-specifi c delivery of a non-polar drug, Trop. J. Pharm. Res., 9, 525–531 (2010). (22) M. K. Robinson, J. P. McFadden, and D. A. Basketter, Validity and ethics of the human 4-h patch test as an alternative method to assess acute skin irritation potential, Contact Dermatitis, 45, 1–12 (2001). (23) J. Fuchs, N. Groth, and T. Herrling, In vitro and in vivo assessment of the irritation potential of differ- ent spin traps in human skin, Toxicology, 151, 55–63 (2000). (24) Y. Obata, Y. Ashitaka, S. Kikuchi, K. Isowa, and K. Takayama, A statistical approach to the develop- ment of a transdermal delivery system for ondansetron, Int. J. Pharm., 399, 87–93 (2010). (25) R. O. Potts and R.H. Guy, Predicting skin permeability, Pharm. Res., 9, 663–669 (1992). (26) H. A. Benson, Transdermal drug delivery: Penetration enhancement techniques, Curr. Drug. Deliv., 2, 23–33 (2005).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)