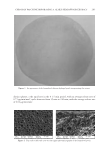
THE ANTI-WRINKLE AND ANTI-MELANOGENIC EFFECTS 275 To exclude the interference of SSE and f SSE in the measurements, wells containing each test agent alone were incubated and reacted with MTT. Absorbance values were normalized to the values obtained for the untreated cells to determine the percentage of survival. ANTIOXIDANT SCAVENGING ACTIVITY The oxygen radical absorbance capacity (ORAC) assay, which measures scavenging activity against peroxy radicals induced by AAPH, was performed as described in a previous report to determine the antioxidant capacities of SSE and f SSE (24). Fluorescein (FL) was used as the fl uorescent probe since the loss of FL fl uorescence is an indication of the extent of damage resulting from its reaction with the peroxy radical. Briefl y, 2 μl of sample or Trolox was incubated with 0.2 mM of β-PE and 200 mM of AAPH in a total volume of 200 μl. To determine decreases in the amount of fl uorescence, fl uorescence was measured at two- minute intervals for 60 min at 37°C. All ORAC analyses were performed on a Synergy HT plate reader at 37°C with an excitation wave length of 535 nm and an emission wave- length of 590 nm. The protective effect of an antioxidant was measured by comparing with a known antioxidant, Trolox, a water-soluble analog of the antioxidative compound, vita- min E. The area under the fl uorescence decay curve (AUC) for FL was calculated as follows: ( ) 1 0 2 0 3 0 19 0 20 0 AUC Areaunderthecurve 1+ f f f f f f f f f f = + + + + "+ ORAC value: sample blank Trolox blank AUC AUC AUC AUC molarityof Trolox molarityof sample ¯ q ¡ ° Final ORAC values were expressed as mean ± SEM. DETERMINATION OF TYROSINASE ACTIVITY Tyrosinase activity was measured using a colorimetric method. Samples were dissolved in DMSO. Each well of the assay plate contained 10 μl of sample with 20 μl of mushroom tyrosinase (1,000 U/ml), and 170 μl of assay mixture (1 mM L-tyrosine, 50 mM phos- phate buffer (PH 6.5), DW= 10:10:9). Reactions were incubated at 25°C for 20 minutes and the absorbance was then measured at 490 nm. Each sample was accompanied by a blank sample that contained all the components except mushroom tyrosinase. The results were compared with a control consisting of DMSO in place of sample. The percentage of tyrosinase activity was calculated as follows: Sample tyrosinse samplealone tyrosinase ¯ q100. ¢ ± HIGH PERFORMANCE LIQUID CHROMATOGRAM ASSAY The Shimadzu LC-20AD HPLC system (Kyoto, Japan) was used with a PDA detector set at 309 nm. The Skypak C18 (250mm × 4.6 mm) was used and temperature was set at 40°C. The gradient for elution consisted of water with 0.5% acetic acid (A) and acetonitrile (B). The
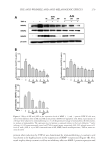
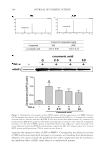
JOURNAL OF COSMETIC SCIENCE 276 mobile phase was used under binary linear gradient conditions as follows: 0–15 min, 10–30% B 15–30 min, 30–60% B 30–35 min, 60–100% B 35–40 min, 100% B 40–41 min, 100–10% B 41–55 min 10% B. The fl ow rate was 1.0 ml/min and the injection volume was 10 μl. SSE (10 mg/ml), f SSE 10 mg/ml), and p- coumaric acid (1,000 μg/ml) were dissolved in methanol using an appropriate dilution. Peaks were identifi ed by com- paring their retention time and UV-vis spectra with p-coumaric acid and were quanti- tated using the corresponding p-coumaric acid curve. STATISTICAL ANALYSIS Data were expressed as means ± SEM. Statistical analyses were performed using the Sigma Plot 12.0 software program. One-way analysis of variance was used to identify signifi - cance ( p 0.05). When signifi cant, specifi c differences between groups were identifi ed using Tukey’s multiple-comparison tests. RESULTS DETERMINATION OF OPTIMAL ETOH EXTRACTION OF SSE AND ITS CYTOTOXICITY To fi nd the optimal EtOH extraction of S. bicolor L. stalks containing high MMP-1 sup- pressing potential, each EtOH fraction of SSE (50 μg/ml) was used to treat NIH-3T3 cells for 24 h. The expression levels of MMP-1 were then determined by immunoblotting. As shown in Figure 1, a 50% EtOH extraction of S. bicolor L. stalk showed the highest suppression of collagen-degrading enzyme MMP-1 expression. In a subsequent experi- ment, SSE lyophilized from a 50% EtOH extraction of S. bicolor L. stalk was used. When the cytotoxicities of SSE and f SSE were assessed, there was no signifi cant cytotoxicity in HDF-N cells treated with up to 100 μg/ml for 24 h (Figure 2). SSE and f SSE resulted in cell cytotoxicity above 200 μg/ml, but the cytotoxicity of f SSE was less than that of its original extract, SSE. These fi ndings suggest that both SSE and f SSE could be produced as effective active ingredients with no associated cytotoxicity when used at concentrations up to 100 μg/ml. MEASUREMENT OF SSE AND FSSE ANTIOXIDANT ACTIVITY Oxidative-stress promotes MMP activity and melanin synthesis, which are causal factors in the induction of skin aging (15). Here, the antioxidant activities of SSE and f SSE were determined using an ORAC assay. As shown in Table I, both SSE and f SSE showed re- markable antioxidant scavenging activity. The antioxidant capacity of SSE at 50 μg/mL was similar to that of Trolox, and f SSE showed higher antioxidant scavenging activity even in lower concentrations compared with SSE. TYROSINASE INHIBITORY EFFECT OF SSE AND FSSE The antioxidant scavenging activities of SSE and f SSE were demonstrated by the ORAC assay (Table I). Skin pigmentation is caused by melanin production mediated by tyrosinase,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)