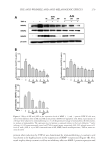
THE ANTI-WRINKLE AND ANTI-MELANOGENIC EFFECTS 273 MATERIAL AND METHODS CHEMICALS AND REAGENTS Water and acetonitrile were high-performance liquid chromatography (HPLC) grade and obtained from Honeywell Burdick & Jackson (Morris Plains, NJ). Arbutin, 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox®), 2,2′-azobis (2-amidinopropane) dihy- drochloride (AAPH), β-phycoerythrin (β-PE), mushroom tyrosinase, L-tyrosine, p-coumaric acid, and tumor necrosis factor-α (TNF-α) were purchased from Sigma (St. Louis, MO). Unless indicated otherwise, all other chemicals were obtained from Sigma. All antibodies were obtained from Cell Signaling Technologies (Beverly, MA). CELL CULTURE NIH-3T3 cells and a melanoma cell line (B16F10) were obtained from the Korean cell line bank (Seoul, Korea) and were maintained in Dulbecco’s modifi ed Eagle’s medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (Gibco) and 1% penicillin (Gibco). Melanoma and fi broblast cells were cultured at 37°C in a humidifi ed atmosphere with 5% CO2. HDF-N cell is a primary human neonatal foreskin cell line and widely used for the wrinkle-repair effi cacy test (19). HDF-N cells were obtained from the Amer- ican Type Culture Collection (ATCC, Manassas, VA) and were maintained in FGM-2 Bullet Kits (Lonza, Switzerland) with 1% penicillin (Gibco) at 37°C in a humidifi ed atmosphere with 5% CO2. For the fermentation of Sorghum bicolor L. stalk (SSE), Aspergillus oryzae (A. oryzae) NK, a widely used fi lamentous fungi in the fermentation process containing abundant digestive enzymes (20), was obtained from Seoul Pharmaceutical (Seoul, Korea) and was cultured at 37°C in Potato Dextrose Broth (BD Biosciences, San Jose, CA) which is used for cultivating yeasts and molds in a shaking incubator. PREPARATION OF THE SORGHUM BICOLOR L. STALK EXTRACT AND ITS FERMENTATION Sorghum bicolor L. Moench stalks were purchased from Danjoungbio (WonJoo, Korea), cut into pieces before their use, and dried in the shade at room temperature. The ethanolic extracts of SSE were extracted for 24 h at room temperature using various concentrations of ethanol ranging from 0 to 100%. Each EtOH extract was then lyophilized and stored for use in subsequent experiments. It has been suggested that the suppressive effect of com- pounds or extracts on MMP-1 protein expression is a good indicator of promising candi- dates (21). NIH-3T3 cells were treated for 24 h with extracts from different concentrations of EtOH, and the cell lysates were then subjected to immunoblotting. Based on the sup- pression of MMP-1 expression (Figure 1), a 50% ethanol extract of SSE was chosen. After the addition of 0.5% A. oryzae NK in 50% SSE, fermentation was carried out at 37°C for 48 h using spinning. The fermented SSE was lyophilized and used in subsequent experi- ments after dissolving in dimethyl sulfoxide (DMSO) at the appropriate concentration. IMMUNOBLOT ANALYSIS Protein extraction and immunoblot were performed as previously described (22). Briefl y, cells were washed twice with cold Dulbecco’s phosphate-buffered saline and then homogenized
JOURNAL OF COSMETIC SCIENCE 274 in the presence of radio immunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), including protease/phosphatase inhibitor cocktails Sigma). Equal amounts of pro- tein (30 μg) were electrophoresed on 12% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane. Binding of each specifi c antibody was visualized using the enhanced chemiluminescence method (Amersham Biosciences, Pittsburgh, PA). Equal loading of samples was confi rmed by reprobing the membranes with anti-β-actin antibody. The signals were detected by UV transilluminators according to the manufacturer’s specifi ca- tions (DAIHAN Scientifi c Co, Seoul, Korea). The band densities from immunoblots were analyzed using Multi Gauge Ver. 3.0 soft- ware (Fujifi lm, Tokyo, Japan). CELL CYTOTOXICITY ASSAY To evaluate the cytotoxicity of SSE and f SSE, a 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide (MTT) assay was performed as described previously with slight modifi cations (23). Briefl y, HDF-N cells (5 × 103 cells/well) were seeded into 96-well plates and incubated overnight. The cells were then treated with various doses of samples for 24 h. Ten microliters of 5 mg/ml MTT solution were added to each well, and the cells were incubated for 4 h. The formazan crystals were dissolved in DMSO, and the absorbance at 540 nm was determined using a Multireader (TECAN, Infi nite 200, Zürich, Switzerland). Fig ure 1. Effect of 50% ethanol extracted SSE on the expression levels of MMP-1 protein. NIH-3T3 cells were exposed to 50 μg/ml extracts of S. bicolor L. stalk produced using different concentrations of EtOH. Equal amount of cell lysate were subjected to immunoblotting. The level of MMP-1 protein is expressed relative to the level of β-actin. Data are the mean ± SEM (n = 3). * indicate -, control similarity. SSE 50% ethanol extract of S. bicolor L. stalk, f SSE A. oryzae NK–fermented form of SSE, MMP matrix metalloproteinase.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)