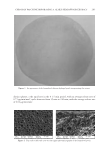
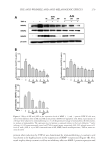
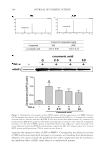
THE ANTI-WRINKLE AND ANTI-MELANOGENIC EFFECTS 281 DISCUSSION In this study, an EtOH extract of S. bicolor L stalks showed antioxidant, antityrosinase, and antiwrinkle activities. These activities of SSE were augmented after fermentation with A. oryzae NK. In f SSE, the amount of p-coumaric acid was increased after fermenta- tion of SSE, and this compound may be, at least in part, associated with the improved actions of f SSE with respect to antiwrinkle activity. Skin aging is a major issue today, involving processes of photo-aging due to industrial pollution and global warming (3). Considering the impacts of having a healthy face and skin in maintaining quality of life, remedies for preventing or suppressing both melano- genesis (through the inhibition of tyrosinase activity) and wrinkle formation mediated by MMPs are of great interest. Sorghum bicolor L. Moench is rich in phytochemicals, such as tannins, phenolic acids, anthocyanins, phytosterols, and policosanols, known to signifi - cantly affect human health, (17). Compared with sorghum grain, the stalk constitutes a much greater proportion of the plant mass and may provide a more cost-effective way of obtaining large quantities of cos- meceutical ingredients. With respect to this, we found that SSE had antioxidant capacity, could suppress both tyrosinase activity (Figure 3) and MMP-1, -2, and -3 protein expression levels (Figure 4), and had additional antioxidant capacity (Table I). To enhance the cos- meceutical effect of SSE, the effect of fermentation of SSE was tested. f SSE showed in- creased antityrosinase activity (Figure 3) and suppressed the protein expression levels of MMP-1, -2, and -3 (Figure 4). Previous reports have suggested that p-coumaric acid possesses antityrosinase and anti- melanogenesis activities because of its similarity to the chemical structure of tyrosine (25,26). SSE containing p-coumaric acid (Figure 5A) showed signifi cant tyrosinase-inhibitory activity however, its reduction potential was relatively less than expected (Figure 3). This low potential of SSE as a tyrosinase inhibitor may be associated with the use of mushroom tyrosinase because the tyrosinase-inhibitory activity of p-coumaric acid is stronger against human tyrosinase than against murine or mushroom tyrosinase. At any rate, the enhanced tyrosinase-inhibitory activity of f SSE may refl ect the increased amount of p-coumaric acid after fermentation (Figure 5A). This result suggests that fermentation of SSE with A. oryzae NK will be effective in enriching the cosmeceutical ingredients in SSE. When we compared the compositions of SSE and f SSE, p-coumaric acid was enriched in f SSE and was able suppress the level of MMP-1 protein. p-Coumaric acid (4-hydroxycinnamic acid) is a phenolic acid found ubiquitously in plants and mushrooms in free or bound forms (27). Numerous activities, including antioxidant, anti-infl ammatory, antimutagenic, antiulcer, antiplatelet, and anticancer activities, associated with p-coumaric acid have been reported (27). In this experiment, the suppressive effect of p-coumaric acid on the expression of MMP-1 protein in HDF-N cells was identifi ed (Figure 5B). However, the elucidation of its under- lying mechanisms will require more extensive work. Further identifi cation of other active compounds involved in the suppression of MMP-1, -2, -3 expression and inhibition of ty- rosinase might be valuable, and thus, additional effort will be required in future experiments. Collectively, SSE possesses antimelanogenic and antiwrinkle activities, and these activities of SSE were further enhanced by fermentation with A. oryzae NK. The p-coumaric acid enriched by fermentation of SSE seems to be one of active compound in SSE. This fi nding may provide insight for the development of cosmeceutical ingredients using the nonedible part of S. bicolor L. Moench.
JOURNAL OF COSMETIC SCIENCE 282 ACKNOWLEDGMENTS This research was supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region (R0000429). REFERENCES (1) M. Ramos-e-Silva and S. C. D. S. Carneiro, Cosmetics for the elderly, Clin. Dermatol., 19(4):413–423 (2001). (2) N. Smit, J. Vicanova, and S. Pavel, The hunt for natural skin whitening agents, Int. J. Mol. Sci., 10(12):5326–5349 (2009). (3) B. Desmedt, P. Courselle, J. O. De Beer, V. Rogiers, M. Grosber, E. Deconinck, and K. De Paepe, Over- view of skin whitening agents with an insight into the illegal cosmetic market in Europe, J. Eur. Acad. Dermatol. Venereol., 30(6):943–950 (2016). (4) G. J. Fisher, H. S. Talwar, J. Lin, and J. J. Voorhees, Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid, Photochem. Photobiol., 69(2):154–157 (1999). (5) H. M. Kim, H. S. An, J. S. Bae, J. Y. Kim, C. H. Choi, J. Y. Kim, J. H. Choi, H. Song, S. H. Moon, Y. J. Park, S. J. Chang, and S. Y. Choi, Effects of palmitoyl-KVK-L-ascorbic acid on skin wrinkles and pigmentation, Arch. Dermatol. Res., 309:397–402 (2017). (6) K. E. Kim, D. Cho, and H. J. Park, Air pollution and skin diseases: Adverse effects of airborne particu- late matter on various skin diseases, Life Sci. 152:126–134 (2016). (7) M. A. Farage, K. W. Miller, P. Elsner, and H. I. Maibach, Characteristics of the aging skin, Adv. Wound Care, 2(1):5–10 (2013). (8) E. Makrantonaki, G. P. Pfeifer, and C. C. Zouboulis, [Intrinsic factors, genes, and skin aging]ssss, Hau- tarzt, 67(2):103–106 (2016). (9) E. D. Lephart, Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms, Ageing Res. Rev. 31:36–54 (2016). (10) M. A. Gupta, A. K. Gupta, N. J. Schork, C. N. Ellis, and J. J. Voorhees, The aging face: A psychocuta- neous perspective, J. Dermatol. Surg. Oncol. 16(10):902–904 (1990). (11) Y. H. Chen, L. Huang, Z. H. Wen, C. Zhang, C. H. Liang, S. T. Lai, L. Z. Luo, Y. Y. Wang, and G. H. Wang, Skin whitening capability of shikimic acid pathway compound, Eur. Rev. Med. Pharmacol. Sci., 20(6):1214–1220 (2016). (12) J. M. Gillbro and M. J. Olsson, The melanogenesis and mechanisms of skin- lightening agents–existing and new approaches, Int. J. Cosmet. Sci. 33(3):210–221 (2011). (13) H. Ando, H. Kondoh, M. Ichihashi, and V. J. Hearing, Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase, J. Investig. Dermatol., 127(4):751–761 (2007). (14) I. F. Videira, D. F. Moura, and S. Magina, Mechanisms regulating melanogenesis, An. Bras. Dermatol., 88(1):76–83 (2013). (15) P. Pittayapruek, J. Meephansan, O. Prapapan, M. Komine and M. Ohtsuki, Role of matrix metallopro- teinases in photoaging and photocarcinogenesis, Int. J. Mol. Sci., 17(6):868 (2016). (16) M. Fanjul-Fernandez, A. R. Folgueras, S. Cabrera, and C. Lopez-Otin, Matrix metalloproteinases: Evo- lution, gene regulation and functional analysis in mouse models, Biochim. Biophys. Acta, 1803(1):3–19 (2010). (17) L. de Morais Cardoso, S. S. Pinheiro, H. S. Martino, and H. M. Pinheiro-Sant’Ana, Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health, Crit. Rev. Food Sci. Nutr., 57(2):372–390 (2017). (18) K. F. Benson, J. L. Beaman, B. Ou, A. Okubena, O. Okubena, and G. S. Jensen, West African Sorghum bicolor leaf sheaths have anti-infl ammatory and immune-modulating properties in vitro, J. Med. Food, 16(3):230–238 (2013). (19) J. E. Lee, I. S. Lee, K. C. Kim, I. D. Yoo, and H. M. Yang, ROS scavenging and anti-wrinkle effects of clitocybin A, isolated from the mycelium of the mushroom Clitocybe aurantiaca, J. Microbiol. Biotechnol., 27:933–938 (2017). (20) R. H. Smith, R. Palmer, and A. E. Reade, A chemical and biological assessment of Aspergillus oryzae and other fi lamentous fungi as protein sources for simple stomached animals, J. Sci. Food Agric., 26(6):785– 795 (1975).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)