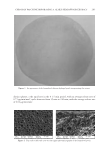
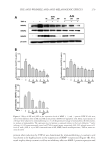
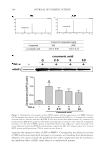
CHITOSAN PATCH INCORPORATING A. ALTILIS HEARTWOOD EXTRACT 267 Table II Measured parameters in the test and control groups. Each value is mean ± SD Parameter Test group (n = 30) Control group (n = 29) Pa % change (test group) % change (control group) Pb Melanin value, AU Week 0 370.8 (2.8) 369.1 (4.1) 0.067 0 0 0 Week 1 367.3 (9.2) 365.3 (3.2) 0.273 2.7 (7.1) 1.5 (4.0) 0.410 Week 3 346.4 (4.4) 364.5 (5.1) 0.001 8.3 (8.9) 1.3 (5.5) 0.001 Week 6 335.8 (8.8) 364.4 (5.9) 0.001 12.5 (12.2) 1.4 (5.2) 0.001 Week 8 317.7 (9.9) 362.8 (2.7) 0.001 19.0 (13.4) 2.3 (4.6) 0.001 Moisture content, AUa Week 0 45.7 (3.3) 46.9 (4.3) 0.233 0 0 0 Week 1 51.3 (1.5) 49.6 (0.9) 0.001 13.2 (18.5) 6.9 (19.4) 0.207 Week 3 51.8 (1.5) 52.3 (1.4) 0.191 20.1 (31.1) 14.7 (28.9) 0.493 Week 6 58.5 (3.1) 53.2 (2.2) 0.001 36.2 (43.8) 16.8 (29.9) 0.053 Week 8 56.4 (2.2) 57.9 (1.7) 0.005 32.2 (45.5) 29.4 (43.6) 0.810 Skin pH Week 0 5.56 (0.04) 5.45 (0.05) 0.001 0 0 0 Week 6 5.45 (0.04) 5.47 (0.04) 0.060 1.7 (4.4) 0.7 (4.7) 0.402 Week 8 5.54 (0.09) 5.32 (0.04) 0.001 0.3 (10.4) 1.7 (10.6) 0.611 Erythema value, AU Week 0 417.7 (3.4) 418.1 (3.4) 0.653 0 0 0 Week 1 421.2 (4.6) 409.3 (4.4) 0.067 1.7 (16.9) 1.0 (11.3) 0.853 Week 3 419.8 (3.3) 408.8 (4.7) 0.059 2.3 (22.4) 1.1 (13.4) 0.805 Week 6 416.3 (3.2) 402.3 (5.1) 0.023 1.4 (17.2) 2.5 (13.8) 0.788 Week 8 414.3 (5.7) 400.4 (4.0) 0.052 0.4 (18.6) 3.7 (12.2) 0.425 Pa: one-way ANOVA compare two mean of each parameter (p 0.05). Pb: one-way ANOVA compare two mean of % change (p 0.05). a One unit represents a water content of stratum corneum of 0.02 mg/cm2. Our previous studies revealed that the surface morphology of the chitosan patch was compact (10,13). However, in the present study, we observed porous structures through- out the formulated patch. It is possible that the presence of other ingredients, such as NaCl in the casting solution interrupted the compactness of the polymeric network of chitosan, resulting in the formation of porosity (17,20). These porous structures infl u- enced the patch fl exibility and the release of the incorporated bioactive components through the patch. We found a high rate and amount of artocarpin release (about 70% of the initial content of artocarpin in the casted patch) in the fi rst 30 min. As the formulated patch was composed of high porous structures, the receptor medium was able to quickly diffuse into the patch. This led to the fast release rate of the artocarpin to the receptor cell (9,21), after which a low amount of artocarpin was released through the patch, because of the low amount of artocarpin remaining in the patch. A 4-h human patch test is an alternative method of determining the acute skin irritation potential of a tested substance (22). When the substance being tested was applied to the skin and then covered with cotton bandage material, such as a Webril pad, the stratum corneum was hydrated. This consequently increased the penetration of the substances through the skin and thereby accelerated the incidence of skin redness and irritation. In the present study, irritation on the volunteers’ forearms was not observed after applying the test patch, whereas skin irritation was clearly observed after applying 20% SLS, which was the positive control for identifying substances or preparations that were classifi ed as
JOURNAL OF COSMETIC SCIENCE 268 irritants (23). When the test patches were applied on subjects’ faces for 8 weeks (three times/week, 30 min for each time), erythema, scaling, and oedema on applied area were not observed, and the skin pH at the applied area was maintained in the normal range (5.5–6.5). These results demonstrate the safe and benefi cial use of the tested patches for use in cosmetics. The outermost layer of the skin is the stratum corneum which plays a role as a barrier layer relying on its lipid and keratin composition and organization. The skin permeation of compounds such as artocarpin, which has a large size (MW 400 dalton) and lipophilic nature (log partition coeffi cient, log P 4) is generally poor (24,25). Reversible alteration of the organization of keratinized protein and/or fl uidization of the lipids by using per- meation enhancers is widely implemented to improve the skin permeation of poor perme- ation compounds. In the present study, the extract enriched with the bioactive compound, artocarpin, was formulated into the hydrogel patch to improve skin permeability of arto- carpin. Water molecules accumulated in the stratum corneum during patch application which contributed to skin moisturization and acted as a permeation enhancer by chang- ing the structure of the keratinized protein in the stratum corneum (26). The microemul- sion nonionic surfactant (Tween® 80) present in the formulated patch also facilitated the permeability of bioactive components by reversely changing the conformation and/or fl uidization of the lipid bilayer (26). We found that the formulated patch containing the extract (0.07 mg of extract/cm2 of patch) signifi cantly improved the hyperpigmented area after 3 weeks of application. This implies that the amount and rate of release of artocarpin from the formulated patch achieved an effective topical delivery, and in addition, a sig- nifi cant increase in the skin moisture content was found after patch application. In conclusion, the artocarpin-enriched A. altilis heartwood extract was formulated into a hydrogel patch by using a microemulaion technique to solubilize the lipophilic extract and by using polymeric chitosan to control the delivery of the artocarpin. The formulated patch was effective in skin depigmentation and was mild on the skin causing no skin red- ness or irritation. However, the small number of subjects in this study did not in- clude the variety of skin types which may be found in studies with a larger more diverse sample. Future studies with a larger number of subjects and a longer duration should be performed to confi rm the benefi cial effects of the formulated patch for improving hyperpigmentation. ACKNOWLEDGEMENTS Financial supports from the Commission on Higher Education, Ministry of Education, Thailand, and International Laboratories Corp. Ltd. We also thank the Center of Excel- lence for Innovation in Chemistry (PERCH-CIC), the S&T Postgraduate Education and Research Development Offi ce (PERDO), the Commission on Higher Education for facil- ity support. Many thanks to Mr. Roy Morien of the Naresuan University Language Cen- tre for his editing assistance and advice on the English expression in this document. REFERENCES (1) M . S. Sikarwar, B. J. Hui, K. Subramaniam, B. D. Valeisamy, L. K. Yean, and K. Balaji, A. review on Artocarpus altilis (Parkinson) Fosberg (breadfruit), J. App. Pharm. Sci., 4, 91–97 (2014).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)