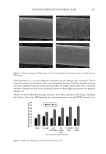
590 JOURNAL OF COSMETIC SCIENCE (Figure 5A), and qPCR revealed that the increase in IL31 mRNA levels was 1.72 ± 0.068-, 2.07 ± 0.208-, 3.03 ± 0.416-, and 1.67 ± 0.153-fold at 6, 12, 24, and 36 h of the control, respectively (Figure 5B). The increase in IL31 protein level was also increased in a time- dependent manner after IL4 stimulation (Figure 5C). In addition, ELISA demonstrated a time-dependent increase in IL31 protein levels secreted into the culture medium by IL4 stimulation (Figure 5D). After 24 h of IL4 stimulation, secreted levels of IL31 peaked, increasing from 23.4 ± 6.96 pg/mL (control) to 113 ± 7.51 pg/mL. These results imply that IL4 stimulates IL31 expression at the transcriptional level in HaCaT cells. We next investigated the effects of the three medicinal herbs on IL4-induced IL31 expression at the transcriptional and translational levels. RT-PCR (Figure 6A) and qPCR (Figure 6B) analyses showed that A houstonianum, B falcatum, and S chinensis significantly (p 0.05) inhibited IL4-induced IL31 mRNA expression in HaCaT cells. Furthermore, their combination treatment inhibited IL4-induced IL31 expression more effectively than each treatment. Similar to mRNA levels, IL4-induced accumulation of IL31 protein was decreased to control levels after treatment with the herbal ethanolic extracts (Figure 6C). As determined by ELISA, pretreatment with each herbal extract significantly (p 0.001) reduced IL31 concentration in the culture medium, and the combination treatment of the three extracts was more effective than their single treatment (Figure 6D). This was supported by the results of double immunofluorescence staining, which showed that IL4 stimulation increased IL31 staining in both the cytoplasm and nucleus. However, exposure to a combination of the three reduced the intensity of IL4-induced IL31 staining, like that of the vehicle control (Figure 7). Our findings on IL31 expression suggest that A houstonianum, B falcatum, or S chinensis inhibit IL4-induced IL31 production and that the combination of the three extracts has a better effect than each extract singly. Figure 5. IL4 upregulates IL31 expression in HaCaT cells (A and B). HaCaT cells were treated with IL4 (20 ng/mL) for various time periods (0–36 h). Total RNA was isolated, and IL31 mRNA levels were examined using RT-PCR (A) and qPCR (B). GAPDH was used as the loading control (C). HaCaT cells were treated as in (B), and whole-cell lysates were immunoblotted using anti-IL31 antibodies. GAPDH was used as the loading control (D). HaCaT cells were treated as in (B), and the concentration of IL31 in the culture medium was determined by ELISA. *p 0.05, **p 0.01, ***p 0.001 compared to control (n = 3).
591 SUPPRESSION OF ITCHING BY THREE HERBAL ETHANOLIC EXTRACTS THREE HERBAL EXTRACTS INHIBIT IL4–INDUCED TSLP EXPRESSION IL4 induces TSLP expression in keratinocytes (49). Epithelial cell-derived TSLP plays a crucial role in initiating Th2 inflammatory responses and contributes to the pathogenesis of various inflammatory disorders, such as AD and asthma (50). TSLP is highly expressed in the skin tissues of patients with AD and model mice with AD-like skin lesions (51). Furthermore, TSLP transmits itch-specific sensitization by directly binding to sensory neurons (52). Therefore, keratinocyte-derived TSLP production is considered a hallmark of itch onset. We observed that IL4 upregulated TSLP expression at the transcriptional and translational levels in a time-dependent manner, as revealed by PCR, RT-PCR (Figure 8A), qPCR Figure 6. Effect of the three herbal extracts on the suppression of IL4-induced IL31 expression in HaCaT cells (A and B). HaCaT cells were treated with IL4 (20 ng/mL) for 24 h in the absence or presence of A houstonianum (40 μg/mL), B falcatum (20 μg/mL), S chinensis (40 μg/mL), or a combination of the three. Total RNA was isolated, and IL31 mRNA levels were examined using RT-PCR (A) and qPCR (B). GAPDH was used as the loading control (C). HaCaT cells were treated as in (A) for 36 h, and whole-cell lysates were immunoblotted using anti–IL31 antibodies. GAPDH was used as the loading control. Relative band intensities were measured using ImageJ (D). HaCaT cells were treated as in (C), and the concentration of IL31 in the culture medium was determined by ELISA. *p 0.05, **p 0.01, ***p 0.001 compared to control (n = 3).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)