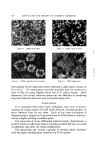
RELATIVE INTENSITY Figure 3. GLYCERYL MONOSTEARATE I00 F FIGURE :3 p GMS 90 I AFTER STORAGE 80 AT S 7O 6O 5O 4O 3O 2O I0 0 18 20 22 24 26 28 30 DEGREES 2 0 GMS after storage at 37øC for 8 •nonths 113 RELATIVE INTENSITY I00 - 90 - 80 70 60 50 40 50 I0- 0 I 18 FIGURE 4 GMS, OLDER MATERIAL AFTER STORAGE 20 22 24 26 28 50 DEGREES 2 O Figure 4. GMS, older material after storage at 37øC for 8 months
114 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS - FIGURE 5A - AS REGEIVED -- -- _ FIGURE 5B _ REHEAT -- -- -- -- i i i i 20 40 60 80 T,øC (CHROMEL:ALUMEL) DIFFERENTIAL THERMAL ANALYSIS HEATING THERMOGRAMS FOR GLYGERY!. IV, Or. STEARATE Figure $•4. Differential thermal analysis heating thermograms for GMS, as received Figure $B. Differential thermal analysis heating thermograms for GMS, reheat SB) although the double exotherm of the cooling cycle is obtained. The sample retains this characteristic for an unknown period of time beyond one week. The temperature of this one complete melt is invariably lower than the temperature of the final melt in the first thermogram. MICROSCOPIC STUDIES A third area of study on GMS involves the examination of samples with the aid of a microscope, particularly as they melt on a hot stage. The general technique which was used utilized the birefringent properties of the crystalline GMS for easy observation. Birefringence is a result of that property of most crystals to rotate the plane of polarized light. As the crystal melts, it becomes morphous and the bright birefringence disappears. The technique is particularly useful in the case of the rela- tively colorless GMS. The microscope and hot stage provided a means of visually observing the same effects that were measured with the more elaborate DTA ap- paratus and X-ray equipment. It was with the microscope that it was first discovered that this brand of GMS actually melted over a wide
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)