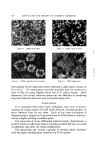
124 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS It can be concluded from these results, in agreement with others (3, 7), that large concentrations of DMSO are required before appreciable en- hancement of penetration can occur. Although Stoughton (1) has re- ported enhanced penetration with relatively low concentrations of DMSO, it is perhaps possible that his experimental procedures allowed for evaporation of water from the treatment solutions thus effectively changing the DMSO concentrations. It was also observed in these experiments that those skin membranes which had been in contact with the greater concentrations of dimethyl sulfoxide appeared more wrinkled and stretched and swollen, yet much less pliable than those skin membranes which had been in contact with buffer or lower concentrations of DMSO. A literature search revealed Table II Reproducibility Study • Initial Concentration Absolute Rate % DMSO (v/v) of Pierate Ion (M) Constant (cm hr -1) 70 3.75 X 10 -2 14.7 X 10 -4 70 3.75 X 10 -2 15.5 X 10 -4 70 3.75 X 10 -• 15.5 X 10 -4 70 3.75 X 10 -• 12.1 X 10 -4 70 3.75 X 10-• 17.6 X 10 -4 Average and standard deviation = 15.1 X 10 -4 4- 2.0 X 10 -4 • (The abdomen of a male guinea pig was wax epilated, and the animal was sacrificed 3 days later by a lethal injection of MgSO4. The abdominal skin was immediately excised and frozen. The length of time between procurement and utilization of the skin was 24-48 hours. The skin was kept frozen until use. ) Table III Effect of DMSO Concentration on Percutaneous Absorption of Pierate Ion s Initial Cone. Approximate % DMSO (v/v) of Pierate (M) Lag Time (hr) 0 4.81 X 10 -• 5.2 20 3.24 X 10-• 5.2 40 5.61 X 10-• 4.9 60 5.15 X 10-2 5.0 72 3.73 X 10-• 4.0 80 6.70 X 10 92 3.53 X 10 • (The abdomen of a male guinea pig was wax epilated, and the animal was sacrificed 3 days later with a lethal injection of MgSO4. The abdominal skin was immediately excised and frozen. The length of time between procurement and utilization of the skin was about 3 days. The skin was kept frozen until use.)
DIMETHYL SULFOXIDE 125 x :• 20 o ) . 0 , 0 20 % DMSO (v/v) Figure 3. Effect of DMSO concentration on percutaneous absorption of pierate ion (pH 7.0, 30 ø 4- 0.05øC) that this observation had been made by others (2, 3). This observa- tion will be treated in greater detail in Part II of this paper. In other experiments, an additional observation was made concern- ing the lag times which are the times necessary for the pierate ion con- centrations in the dermal chambers to increase in a linear manner with time. With increasing concentrations of DMSO, the lag times generally decreased. While these lag times do not enter into the calculation of the absolute rate constant, they could be indicative of the rate of some al- teration in the diffusion process brought about by DMSO. These re- suits are shown in Table III. Since the experiments described above involved establishing a large concentration gradient for DMSO across the skin membrane, it was de- cided to study whether this concentration gradient was important for the enhanced percutaneous penetration of pierate ion. Experiments were set up in which the penetration of pierate was studied in the absence of DMSO with DMSO on the epidermal side of the skin membrane and buffer on the dermal side and with DMSO on both sides of the skin membrane. The results shown in Table IV dem- onstrate that the enhanced penetration of pierate is not altered by eliminating the concentration gradient for DMSO across the skin. In order to establish conclusively that DMSO does not "carry" pierate through the skin, experiments were performed in which the rates
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)