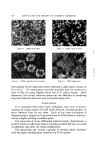
GLYCERYL MONOSTEARATE 117 The implications of this work are also significant because this change in form can occur when GMS is in combination with other constituents. It is also known that similar behavior can be observed of other stearate esters. Because of the wide use of these compounds, the importance of a knowledge of this behavior is obvious. Hoerr has stated in a paper (2) his belief that the various polymorphs and sub forms can be explained on a basis of purity of material. He sug- gests that the keterogeneeus •ature of these compounds retards crystalli- zation, but eventually, given suitable conditions, the crystallization will take place. This description seems to fit the observations reported here, particu- larly in the microscopic examination of mixtures of GMS with other components. Here one can actually see the growth of crystals and ap- parently at the expense of the surrounding material. Under such circumstances, the crystallization theory seems more appropriate than the conversion of a mass of material from one form to another. From this theory one can speculate that certain constituents might be isolated and identified which could retard crystallization better than others. This should be a fruitful area of research. The techniques and instrumentation for the analytical work are available aS never be fore. A combination of these assets and experience in emulsion tech- nology should produce valuable data for a wide variety of users. (Received May 19, 1967) RI•FERENCES (1) Kuhrt, N.H., Broxholm, R. A., and Blum, W. P., Conjoined crystals. I. Composition and physical properties, J. Am. Oil Chemists' Soc., 4,725-733 (1963). (2) Hoerr, C. W., X-ray diffraction of fats, Ibid., News Ed., 41, 4 (1964).
J. Soc. Cosmetic Chemists, 19, 119-127 (Feb. 5, 1968) The Effect of Dimethyl Sulfoxide on Percutaneous Absorption: A Mechanistic Study, Part I STANLEY G. ELFBAUM, Ph.D., and KARL LADEN, Ph.D.* Presented before the Mid-Atlantic Chapter, February 14, 1•67 Synopsis--An in vitro skin penetration system has been described in which intact abdominal guinea pig skin has been utilized as the membrane. The passage of pierate ion through this membrane in the presence of dimethyl sulfoxide (DMSO) is a passive diffusion process which shows adherence to Fick's First Law of Diffusion. In order to produce substantial enhance- ment effects, relatively large concentrations of DMSO were required. Effective concentra- tions of DMSO caused the skin membranes to acquire a more turgid and wrinkled appearance. It has been shown by diffusion and isotope techniques that the absolute rate constant for the penetration of DMSO is approximately 100 times greater than that for the pierate ion. Thus, the two substances transferred independently of each other through the skin. INTRODUCTION In recent years, dimethylsulfoxide (DMSO) has been reported as a solvent which, in addition to having a host of claimed therapeutic properties, has the ability of rapidly penetrating human skin and en- hancing the percutaneous absorption of materials dissolved therein (1-7). While there have been numerous reports concerning the utility of DMSO in promoting percutaneous absorption both in vivo and in vitro, few studies have appeared concerning its mechanism of action upon the barrier to absorption through the skin (7). Recently, disclosure of potential medical hazards associated with the use of DMSO has precluded its widespread use in humans (6). How- * Gillette Research Institute, 6220 Kansas Avenue, N. E., Washington, D.C. 20011. 119
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)