620 K. C. James and E. Tsirivas It thus appears that the analytical procedure described can be used for the analysis of hydrogen peroxide emulsions, subject to prior standardisation of the method to the paxticular formula. Preparations containing cationics should be treated with caution however. The potential of ethyl acetate as extracting solvent was also investigated. It could be used satisfactorily in the determination of hydrogen peroxide in aqueous solutions, but the results were sensitive to the presence of every surface active agent examined. REFERENCES 1 Kingzett, C. T. Report on the atmospheric oxidation of phosphorus, and some reactions of ozone and hydric peroxide. J. Chem. Soc. 792 (1880). 2 Henderson, G. and Newton, J. M. The solubilization of iodine by a non-ionic surfactant. Pharm. Acta. Helv. 41 228 (1966). 3 Henderson, G. and Newton, J. M. The antibacterial activity of iodine in aqueous solutions of a non-ionic surfactant. Pharm Acta. Helv. 44 129 (1969). 4 Hickman, J. B. Determination of hydrogen peroxide in the presence of organic matter. Proc. W. Va. Academy Sci. 23 76 (1951). 5 Dukes, E. K. and Hyder, M. L. Determination of peroxide by automatic colorimetry. Anal. Chem. 36 1698 (1964). 6 Ovenston, T. C. J. and Rees, W. T. The spectrophotometric determination of small amounts of hydrogen peroxide in aqueous solution. Analyst 75 204 (1950). 7 Azaz, E., Donbrow, M. and Hamburger, R. Assay of micro-scale amounts of hydroperoxide in aqueous non-ionic surfactant solutions by a spectrophotometric method. ibid. 98 663 (1973). 8 Evans, D. F. Blue perchromic acid. J. Chem. Soc. 4013 (1957). 9 Glasner, A. and Steinberg, M. Photometric determination of chromium as perchromic acid in ethyl acetate solution. Anal. Chem. 27 2008 (1955). 10 Sastri, M. N. and Sundar, D. S. Quantitative extraction of chromium as blue perchromic acid with tri-n-butyl phosphate. Z. Anal. Chem. 195 343 (1963). 11 Tuck, D. G. A solvent extraction method for the determination of microgram amounts of chromium. Anal. Chim. Acta 27 296 (1962). 12 Haggett, M. L., Jones, P. and Wynne-Jones, W. F. K. Peroxycomplexes as intermediates in the catalytic decomposition of hydrogen peroxide. Diss. Faraday Soc. 29 153 (1960). 13 Cosmetic and pharmaceutical formulary. Croda. P. 6. 14 Suggested formulae for the manufacture of pharmaceutical and cosmetic preparations based on Dehydag products. Henkel & Cie, Dusseldorf. p. 153.
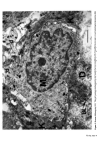
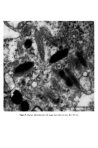
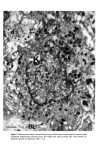
J. Soc. Cosmet. Chem. 28 621-627 (1977)¸ 1977 Society of Cosmetic Chemists of Great Britain Iauses of skin 0olouration, origin, development and struoture of pigment oells J. A. A. HUNTER Department of Dermatology, The Royal Infirmary, Edinburgh Presented at the Joint Symposium with the Pharmaceutical Society of Great Britain, "Cosmetic and Pharmacological Aspects of Colour" 9-11 November 1976, Stratford upon Avon Synopsis Haemoglobin, oxyhaemoglobin, melanin and carotene are pigments responsible for the colour of human skin. An abnormal skin colour is produced either by an imbalance in the proportion of these four pigments or by an abnormal pigment. Melanin is synthesised in melanoeytes found usually in the basal layer of the epidermis. Within the melanocytes melanin is bound to a protein matrix and the melanosomes so formed are transferred to surrounding keratinocytes. The number of melanocytes is similar in Caucasoid and Negroid skin. Black skin is produced by increased melanocytic activity associated with the production of melanosomes which are larger than those in Caucasold skin. Negroid melanosomes tend to be disposed individually in keratinocytes whereas those in Caucasoids are usually complexed. INTRODUCTION During the last decade considerable progress has been made in understanding mechanisms of skin colouration, particularly the formation and distribution of melanin. In this paper an attempt will be made to review the topic and to emphasise views which have been generally accepted. SKIN PIGMENTS Human skin varies in thickness from about 3 to 5 mm and consists of three main layers: a stratified squamous epithelium on the surface, called the epidermis a connective tissue dermis, and an underlying fatty layer. Combinations of four pigments (Table I) are responsible for the various colours of human skin. Table I. Pigments concerned in normal skin colour Haemoglobin Oxyhaemoglobin Melanin Carotene 621
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)