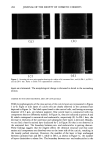
340 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS thenylethylether, was hardly detectable even at its protonated molecular ion (m/z 170), while pantothenylethylether was detected with more than sufficient S/N (Figure 7). In Figure 8, the mass spectrum of the 6.7 min peak is shown. A protonated molecular ion (234, M + H) and a methanol adduct ion (266) were found, together with fragment ions at m/z 104, 188, and 218. Mass chromatograms of standard solutions of pantothenyl- ethylether are shown in Figure 9. The calibration curve (data not shown) showed excellent linearity with a correlation coefficient of 0.9993. The result obtained, shown in Figure 10, was in good agreement with the actual amount (0.2%). Two nanograms m/z: •..800 ng Std, 1 1,0 ng •E+06 i: 88 5:30 6: oo 6:30 7:88 7:38 8:88 8:38 9: oo Std, 2 2,0 ng 9:30 i0:88 •E+06 .00• :8{) 5:30 6:{)0 6: 3{) 7: 8{) 7:38 8:{)8 8:38 9: 8{) 9:30 -2. 888 (d,) / ng ' •' '1 ' • '' ' I ' ' ' ' ' I ' '""' I •'1 ' I ' ' ' ' ' I ' I ' I ' 5: 30 6: 00 6: 30 7: 08 7: 38 8: 00 8: 30 9: 80 9: 30 Figure 9. Mass chromatograms of standard solutions of pantothenylethylether. Methyl p-hydroxybenzoate (a) (internal standard, I.S.), 10 ng and pantothenylethylether (b) 1.0 ng, (c) 2.0 ng, and (d) 4.0 ng.
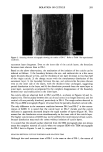
ANALYSIS OF COSMETIC INGREDIENTS 341 •E+Q6 .000 5:30 6:00 6:30 7:00 7:30 •_,: •o 8' 30 9:00 9:30 Figure 10. Mass chromatogram of pantothenylethylether in hair lotion. The result obtained was in good agreement with the actual amount (0.2%). of pantothenylethylether was introduced into the mass spectrometer, and the coefficient of variation of the peak area ratio was 1.3% at this amount for five repeated analyses. CONCLUSION Semi-micro HPLC/MS with a reversed-phase column using a methanol-and-water mix- ture as the mobile phase, and with post-column adjustment of methanol concentration at the ion source, was successfully applied to the quantitative analysis of pantothenyl- ethylether in hair lotion. Results obtained with 30 cosmetic ingredients suggest that it will be possible to apply this system after further development to the analysis of many ingredients in cosmetics. REFERENCES (1) S. Scalia, F. Testoni, G. Frisina, and M. Guarneri, Assay of 1,4-dioxane in cosmetic products by solid-phase extraction and GC-MS, J. Soc. Cosmet. Chem., 43, 207-213 (1992). (2) E. Gmahl and W. Ruess, Identification and characterization of vinylpyrrolidone-vinylimidazolium chloride copolymers in cosmetic products by pyrolysis-gas chromatography-mass spectrometry method, Int. J. Cosmet. Sci., 15, 77-81 (1993). (3) R. M. Caprioli, T. Fan, andJ. S. Cottrell, Continuous-flow sample probe for fast atom bombardment mass spectrometry, Anal. Chem., 58, 2949-2954 (1986). (4) U. Justesen and G. Bojesen, Analysis of some eicosanoids by continuous-flow fast atom bombardment mass spectrometry, J. Chromatogr., 562, 59-66 (1991). (5) P. Kokkonen, W. M. A. Nissen, U. R. Tjaden, and J. van der Greef, Bioanalysis of erythromycin 2'-ethylsuccinate in plasma using phase-system switching continuous-flow fast atom bombardment liquid chromatography-mass spectrometry, J. Chromatogr., 565, 265-275 (1991). (6) M. Suzuki, T. Yamakawa, and A. Suzuki, A micro method involving micro high-performance liquid chromatography-mass spectrometry for the structural characterization of neutral glycosphingolipids and monosialogangliosides, J. Blochem., 109, 503-506 (1991). (7) J. A. Page, M. T. Beer, and R. Lauber, Optimization of continuous flow fast atom bombardment mass spectrometry for bioanalysis, J. Chromatgr., 474, 51-58 (1989). (8) Y. Ikarashi and Y. Maruyama, Applicability of high-performance liquid chromatography-continuous- flow fast atom bombardment mass spectrometry for simultaneous quantitation of multiple neuro- chemicals, J. Chromatgr., 587, 306-313 (1991).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)