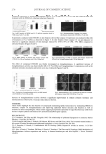
JOURNAL OF COSMETIC SCIENCE 240 produces trehalose glasses in situ at their current locations. The trehalose glasses formed pick up water as the humidity rises and thus reduce the amount of moisture available to disturb the style. Furthermore, the trehalose glasses may act as pore blockers and steri- cally hinder water from reaching certain parts of the hair. Finally, molecular trehalose may itself stabilize the styled confi guration through effects related to hair protein bind- ing (28) and/or structuring water (29). The striking anti-humidity benefi ts as demonstrated in this paper may be a result of one or more mechanisms described above. More work needs to be done to completely resolve the mechanism of action. In summary, here we see a macroscopic anti-humidity benefi t at the hair array level that seems intimately related to the water uptake and solid state polymorphism of trehalose. Clearly, glassy trehalose regulates moisture in the fi ber and affects the viscoelastic behavior and subsequent aging characteristics of the hair polymer in some way that, though not fully understood, seems to be related to the water uptake properties of this form of trehalose. We think that the presence of the trehalose glass and its ability to regulate moisture in the hair is the major contributing factor for the anti-humidity effect. CONCLUSION Hair treated with trehalose and heat-straightened shows long-lasting hair style reten- tion even at high humidity compared to hair treated with water, provided that the conditions at the style creation stage are suitable, i.e., low-humidity (50%) condi- tions. The formation of trehalose glasses in situ during the heat straightening process on hair treated with trehalose may be responsible for the anti-humidity effect seen. Treha- lose glasses regulate moisture in the fi ber at high humidity, giving longer-lasting style benefi ts. ACKNOWLEDGMENTS The authors thank Janet Cotterall for the initial switch work. REFERENCES (1) N. K. Jain and I. Roy, Effect of trehalose on protein structure, Protein Sci., 18, 24–36 (2009). (2) A. D. Elbein, Y. T. Pan, I. Patuszak, and D. Carroll, New insights on trehalose: A multifunctional mole- cule, Glycobiology, 13(4), 17R–27R (2003). (3) J. G. Streeter, Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation, J. Appl. Micro- biol., 95, 484–491 (2003). (4) N. Benaroudj, D. H. Lee, and A. L. Goldberg, Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals, J. Biol. Chem., 276(26), 24261–24267 (2001). (5) Y. Nie, J. J. de Pablo, and S. P. Palecek, Platelet cryopreservation using a trehalose and phosphate formulation, Biotech. Bioeng., 92(1), 79–90 (2005). (6) J. H. Crowe, L. M. Crowe, A. E. Oliver, N. M. Tsvetkova, W. F. Wolkers, and F. Tablin, The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state, Cryobiology, 43, 89–105 (2001). (7) W. F. Wolkers, N. J. Walker, Y. Tamari, F. Tablin, and J. H. Crowe, Towards a clinical application of freeze-dried human platelets, Cell Preserv. Technol., 1(3), 175–188 (2003). (8) J. K. Kaushik and R. Bhat, Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose, J Biol. Chem., 278(29), 26458– 26465 (2003).
TREHALOSE IN HAIR CARE 241 (9) S. Ohtake and Y. J. Wang, Trehalose: Current use and future applications, J Pharm. Sci., 100(6), 2020–2053 (2011). (10) GNPD or Global New Products Database (www.gnpd.com) lists many hair products containing trehalose from companies such as Unilever, Avon, Redken, etc. (11) M. Nakagaki, H. Nagase, and H. Ueda, Stabilization of the lamellar structure of phosphatidylcholine by complex-formation with trehalose, J. Membr. Sci., 1328, 197–206 (1992). (12) H. Yoshii, T. Furuta, J. Kudo, and P. Linko, Crystal transformation from anhydrous alpha-maltose to hydrous beta-maltose and from anhydrous trehalose to hydrous trehalose, Biosci. Biotechnol. Biochem., 64, 1147–1152 (2000). (13) R. Surana, A. Pyne, and R. Suryanarayanan, Effect of preparation method on physical properties of amorphous trehalose, Pharm. Res., 21, 867–874 (2004). (14) D. J. Van Drooge, W. L. J. Hinrichs, and H. W. Frijlink, Incorporation of lipophilic drugs in sugar glasses by lyophilization using a mixture of water and tertiary butyl alcohol as solvent, J. Pharm. Sci., 93(3), 713–725 (2004). (15) F. J. Wortmann, M. Stapels, and L. Chandra, Modeling the time-dependent water wave stability of human hair, J. Cosmet. Sci., 61(1), 31–38 (2010). (16) F. J. Wortmann, M. Stapels, and L. Chandra, Humidity-dependent bending recovery and relaxation of human hair, J. Appl. Poly. Sci., 113(5), 3336–3344 (2009). (17) E. H. Hunter, C. S. Frampton, D. Q. M. Craig, and P. S. Belton, The use of dynamic vapour sorption methods for the characterization of water uptake in amorphous trehalose, Carbohydrate Res., 345, 1938–1944 (2010). (18) A. Moran and G. Buckton, Studies of the crystallization of amorphous trehalose using simultaneous gravimetric vapor sorption/near IR (GVS/NIR) and “modulated” GVS/NIR, AAPS PharmSciTech, 10(1), 297–302 (2009). (19) K. Keis, C. L. Huemmer, and Y. K. Kamath, Effect of oil fi lms on moisture vapor absorption on human hair, J. Cosmet. Sci., 58, 135–145 (2007). (20) J. H. Crowe, L. M. Crowe, and D. Chapman, Preservation of membranes in anhydrobiotic organisms—The role of trehalose, Science, 223, 701–703 (1984). (21) C. W. B. Lee, S. K. Dasgupta, J. Mattai, G. G. Shipley, O. H. Abdel-Mageed, A. Makriyannis, and R. G. Griffi n, Characterization of the L-lambda phase in trehalose-stabilized dry membranes by solid state NMR and X-ray diffraction, Biochemistry, 28, 5000–5009 (1989). (22) J. H. Crowe, L. M. Crowe, J. F. Carpenter, and C. Wistrom, Stabilization of dry phospholipid bilayers and proteins by sugars, Biochem. J., 242, 1–10 (1987). (23) J. F. Carpenter and J. H. Crowe, An infrared spectroscopic study of the interactions of carbohydrates with dried proteins, Biochemistry, 28, 3916–3922 (1989). (24) A. H. Haines, Non-equivalence of D- and L-trehalose in stabilising alkaline phosphatase against freeze- drying and thermal stress. Is chiral recognition involved? Org. Biomol. Chem., 4, 702–706 (2006). (25) J. L. Green and C. A. Angell, Phase relations and vitrifi cation in saccharide-water solutions and the trehalose anomaly, J. Phys. Chem., 93, 2880–2882 (1989). (26) H. Levine and L. Slade, Another view of trehalose for drying and stabilizing biological materials, Biopharm., 5, 36–40 (1992). (27) K. L. Koster, M. S. Webb, G. Bryant, and D. V. Lynch, Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: Vitrifi cation of sugars alters the phase behavior of the phospholipid, Biochim. Biophys. Acta, 1193, 143–150 (1994). (28) T. Furuki, K. Oku, and M. Sakurai, Thermodynamic, hydration and structural characteristics of alpha, alpha-trehalose, Frontiers Biosci., 14, 3523–3535 (2009). (29) Q. Liu, R. K. Schmidt, B. Teo, P. A. Karplus, and J. W. Brady, Molecular dynamics studies of the hydration of alpha, alpha-trehalose, J Am. Chem. Soc., 119(33), 7851–7862 (1997).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)