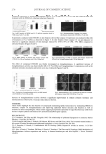
JOURNAL OF COSMETIC SCIENCE 226 Tyrosinase is responsible for enzymatic browning in plants and therefore is considered to produce undesirable changes in color, fl avor, and nutritive values of plant-derived foods (2,3). Therefore, tyroinase inhibitors may prevent the browning reaction caused by ty- roinase and maintain the appearance of plant foods. Furthermore, tyrosinase catalyzes the reaction of melanin biosynthesis in human skin and results in dark skin (4). Recently, safe and effective tyrosinase inhibitors have become im- portant for their potential applications in preventing pigmentation in human beings (1,4,5). Therefore, tyrosinase inhibitors are also important in cosmetic applications for skin-whitening effects, since certain people prefer a lighter skin color (6). Plants are rich sources of bioactive chemicals and mostly free from harmful side effects, and there is an increasing interest in fi nding natural tyrosinase inhibitors from them. Some potent tyrosinase inhibitors, such as cuminaldehyde (7), oxyresveratrol (8), kaemp- ferol (9), quercetin (10), and gallic acid derivatives (11) have been isolated from various plants. Phenolic compounds are rich in many plants and they have been shown to possess anti-tyrosinase activity (10–13). In this study, the effects of ethanol extracts from the peels and the seeds of two grape cultivars, Kyoho grapes and Red Globe grapes, on mushroom tyrosinase activity were investigated. MATERIALS AND METHODS CHEMICALS Mushroom tyrosinase (4,000 units/mg), and 3,4-dihydroxyphenylalanine (L-dopa) were purchased from Sigma Chemicals Co. (St. Louis, MO). HPLC-grade ellagic acid, gallic acid, cinnamic acid, resveratrol, and catechin hydrate were also purchased from Sigma. Chlorogenic acid and kuromanin chloride were purchased from Extrasynthese (Genay, France). Kyoho grapes (Vitis vinifera × Vitis labrusca) and Red Globe grapes (Vitis vinifera) were purchased from a local farmer in Taichung city, Taiwan, ROC. PREPARATION OF ETHANOL EXTRACT FROM THE PEEL AND THE SEED OF GRAPES Grape peel and seed were homogenized with 95% ethanol (1: 10, weight (g)/volume (ml)) for 1 min and then set in a refrigerator at 4°C for 12 h. After centrifuging the mixture at 7000g at 4°C for 20 min, the ethanolic solution was fi ltrated with Whatman No.l paper, and then ethanol was removed in an evaporator at a temperature lower than 40°C. MEASUREMENT OF TOTAL PHENOLIC CONTENT The total phenol content was measured using Folin-Ciocalteu’s reagent method (14). The sample (0.5 ml, 200 mg sample/ml) was mixed with 0.5 ml of Folin-Ciocalteu’s reagent for 3 min and then mixed with 0.05 ml of 10 % Na2Co3. The absorbance of the mixture was measured at 735 nm after 1-hr incubation at room temperature. Gallic acid was used as the
ANTI-TYROSINASE ACTIVITY OF ETHANOL FROM GRAPES 227 standard for the calibration curve, and the total phenolic content was expressed as gallic acid equivalents (mg/g dry material). CHARACTERIZATION OF PHENOLIC COMPOUNDS IN EXTRACTS Phenolic compounds in the tested extract were analyzed based on the method described by Li et al. (15), with a slight modifi cation. Dried extract (10 mg) was dissolved in l ml of 0.1% phosphoric acid, fi ltered through a 0.45-μm fi lter, and analyzed by HPLC. HPLC analysis was performed using a Hewlett- Packard HPLC system (HP 1100 series, Waldron, Germany), consisting of a quaternary pump and a variable wavelength detector (VWD) at 270 nm and equipped with a Li-Chrospher RP-18 cartridge column (Merck, 250 mm × 4.6 mm, 5 μm). The mobile phase was a stepwise gradient of water (0.1% v/v phosphoric acid)–acetonitrile (0.01 min, 100:0 50 min, 20:80), and the injection volume was 30 μl. The identifi cation of each compound was based on a combination of retention time and spectral matching by comparison with those of known standards. ENZYMATIC ASSAY OF TYROSINASE The tyrosinase activity using L-dopa as substrate was measured according to the method of Kubo and Kinst-Hori (7), with slight modifi cations. First, 0.29 ml of 4.5 mM L-dopa solu- tion (the substrate for tyrosinase) was mixed with 0.3 ml of 25 mM phosphate buffer (pH 6.8) and incubated at 25°C for 10 min. Then, 0.3 ml of tested samples of different concen- trations (1, 5, 10, and 15 mg/ml) was added to the mixture followed by the addition of 0.01 ml of 4000 units/ml mushroom tyrosinase. The formation of dopachrome was immediately monitored by measuring the linear increase in optical density at 475 nm. Triplicate measurements were recorded. The increased absorbance at 475 nm was recorded during 10 min at room temperature. Deionized water was used instead of the extract for the blank. One unit (U) of enzymatic activity was defi ned as the amount of enzyme needed for increasing 0.001 absorbance per min at 475 nm under the experimental conditions. DETERMINATION OF KINETIC PARAMETERS Mushroom tyrosinase (0.03 ml, 4000 units/ml) was incubated with various concentra- tions of 0.27-ml enzyme substrates (L-dopa, 0.6–0.66 mg/ml) in 0.3 ml of 25-mM phos- phate buffer (pH 6.8) at room temperature, and tested samples (0.3 ml) were added to the reaction mixture simultaneously. The kinetic parameters, Km and Vmax, of the tyrosinase activity were calculated by linear regression from Lineweaver-Burk plots. STATISTICAL ANALYSIS For each measurement, three replicates were conducted. The data were presented as the mean ± standard deviation. One-way analysis of variance (ANOVA) was conducted using a package (SAS Institute Inc., Cary, NC). Duncan’s multiple ranges test was used to de- termine the signifi cant difference between different treatments.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)