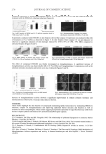
JOURNAL OF COSMETIC SCIENCE 230 gallic acid equivalent/g (Table I). Our data indicated that the total phenolic content in ethanol extracts from grape seeds was much higher than in grape peels. The amount of phenolic compounds in the various grape derivatives parallels the anti-tyrosinase activity. Therefore, it was quite possible that phenolic compounds contributed to the anti-tyrosinase activity in the grape extracts. As the anti-tyrosinase activity in the seed extracts are the main interest, it was noted that even though KG-SEE had a higher total phenolic content than RGG-SEE, the anti- tyrosinase activity of KG-SEE was lower than that of RGG-SEE. This indicated that total phenolic content was not the only factor affecting the anti-tyrosinase activity, and that the profi le of phenolic compounds in the extracts might also be an important variable. The phenolic compounds in KG-SEE and RGG-SEE were determined by using HPLC, and the results are shown in Table II. Among the detected phenolic compounds, there were four compounds that showed a signifi cant difference between KG-SEE and RGG-SEE. RGG-SEE had higher gallic acid and kuromanin chloride content than KG-SEE, but lower catechin hydrate and ellagic acid content. After testing the anti-tyrosinase activity of these four pure phenolic compounds, we found that the anti-tyrosinase activity fol- lowed the order: gallic acid catechin hydrate kuromanin chloride ellagic acid (Figure 4). Table I Total Phenolic Content in the Ethanol Extracts from the Peels and Seeds of Kyoho Grapes and Red Globe Grapes* KG-SEE KG-PEE RGG-SEE RGG-PEE 400±11a 18±1d 339±7b 27±0c *Total phenolic content is presented as the amount of gallic acid equivalent (mg gallic acid equivalent/g). a,b,c,d The values in the same row followed by different superscripts were signifi cantly different ( p 0.05). Table II Phenolic Compounds in the Ethanol Extracts from the Seeds of Kyoho Grapes and Red Globe Grapes Determined by HPLC Compounds Content (g kg−1) KG-SEE RGG-SEE Gallic acid 2.22b 4.60a Catechin hydrate 5.49a 2.27b Kuromanin chloride — 2.75 Ellagic acid 1.61a 0.05b Flavon 0.60* 0.65 Rutin — — Chlorogenic acid — — Resveratrol — — Quercetin — — Cinnamic acid — — —Not detected. *The values in the same row are not signifi cantly different. a,b The values in the same row followed by different superscripts are signifi cantly different ( p0.05).
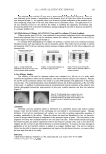
ANTI-TYROSINASE ACTIVITY OF ETHANOL FROM GRAPES 231 Figure 4. Effect of pure phenolic compounds on mushroom tyrosinase activity using L-dopa (0.33 mg/ml) as the substrate. Mushroom tyrosinase (44 units/ml) reacted at room temperature for 10 min. Symbols: - - (no inhibitor) - - (ellagic acid 0.03 mg/ml) -▲- (gallic acid 0.03 mg/ml) - - (kuromanin chloride 0.03 mg/ml) -×- (catechin hydrate 0.03 mg/ml). Gallic acid has been identifi ed as a tyrosinase inhibitor from many plants, and it inhibits the diphenolase activity of mushroom tyrosinase (18,19). Since the gallic acid content in RGG-SEE was about twice that in KG-SEE and gallic acid showed high anti-tyrosinase activity, we suggested that gallic acid was the main compound affecting the anti-tyrosinase activity in the ethanol extracts from grape seeds. CONCLUSION Grape seeds had higher anti-tyrosinase activity than grape peels because grape seeds had higher total phenolic compounds. The profi le of phenolic compounds was also an impor- tant factor affecting anti-tyrosinase activity. It was suggested that gallic acid was the main compound affecting the anti-tyrosinase activity in the ethanol extracts from grape seeds. The inhibitory mechanism of the ethanol extracts from the grape seeds was a mix-type inhibition. Since grape seeds were the potential source of the tyrosinase inhibitor, they might serve as a skin-lighting agent. ACKNOWLEDGMENT This study was supported by the National Science Council (NSC 97-2313-B-468- 002-MY3), Taiwan, ROC. Their fi nancial support is greatly appreciated. REFERENCES (1) S. Y. Seo, V. K. Sharma, and N. Sharma, Mushroom tyrosinase: Recent prospects, J. Agr. Food Chem., 51, 2837–2853 (2003). (2) A. Sánchez-Ferrer, J. N. Rodríguez-López, F. García-Cánovas, and F. García-Carmona, Tyrosinase: A comprehensive review of its mechanism, Biochim. Biophys. Acta, 1247, 1–11 (1995).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)