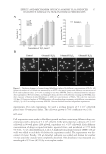
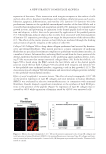
JOURNAL OF COSMETIC SCIENCE 38 HOECHST 33342 STAINING According to the instructions of the Hoechst 33342 staining kit, fi broblasts were plated onto the glass coverslips at a density of 1 × 104 cells/well, and left overnight. The adher- ent cells were pretreated with 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 mmol/l H2O2 to prepare the H2O2-induced cell injury model. After 3-, 6-, 12-, and 24-h treatment, the culture me- dium was discarded and fi xed with 4% paraformaldehyde for 20 min. The fi xative solu- tion was discarded and rinsed 3 times every 5 min with phosphate-buffered saline (PBS). Figure 2. Effect of EGCG on H2O2-induced dermal fi broblast injury. (A) Statistical analyses of the effect of EGCG on viability rates of human dermal fi broblasts undergoing concentration of 0–200 μg/ml with H2O2 injury. The viability rates were measured with an MTT colorimetric assay. *p 0.05 **p 0.01 according to the Stude nt’s t-test compared with H2O2 alone. Data are obtained from fi ve independent experiments. (B) Effect of EGCG on H2O2-induced apoptosis detected by the TUNEL assay. (C) Statistical analysis of the effect of EGCG on the apoptotic rate induced by H2O2. *p 0.05 by Student’s t-test, compared with H2O2 alone.
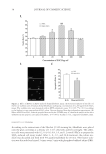
EFFECT AND MECHANISM OF EGCG AGAINST H2O2-INDUCED OXIDATIVE DAMAGE IN HUMAN DERMAL FIBROBLASTS 39 Finally, 1 ml of Hoechst 33342 was added per well, stained for 20 min, and rinsed 3 times with PBS. Coverslips on slide containing antiquench ingredients were sealed and protected from light. The nucleus can be visualized with a fl uorescence microscope. TUNEL STAINING The TUNEL was used to detect the apoptosis of human dermal fi broblasts. Cells were plated onto 35-mm petri dishes, fi xed in 4% paraformaldehyde for 25 min at 4°C, and permeabilized with 0.1% Triton-X-100/PBS. Fluorescein isothiocyanate (FITC)-labeled fl uorescein nucleotide and terminal deoxynucleotidyl transferase (TDT) mixture were then added for 60 min at 37°C. When visualized under a fl uorescence microscope, the FITC- stained nucleus was scored as a positive result. Negative control group was used without TDT solution. Counting was based on fi ve randomly selected areas across horizon, and the apoptotic rate is equal to the number of green fl uorescent cells divided by the total num- ber of living cells and the green fl uorescent cells. ASSAY FOR DPPH RADICAL SCAVENGING ACTIVITY Samples (1 ml) at different concentrations of EGCG and control were added to 1 ml of DPPH solution (12 mg/100 ml). Ultrapure water was added at a fi nal volume of 4 ml, shaked vigorously for 30 s, and left in a dark room for 5 min at room temperature. Its absorbance was recorded at 517 nm. The effect of DPPH radical scavenging of the sample was calculated according to the following formula: DPPH scavenging effect (%) = {1 − (T − T0)/(C − C0)} × 100%, where T0 is the absorbance of the contrast sample, T is the absorbance of the sample, C is the absorbance of the positive control, and C0 is the absorbance of the negative control. DETERMINATION OF SOD, GSH-PX, AND MDA LEVELS The concentration of SOD and the activities of MDA were determined using commer- cially available kits purchased from the Jiancheng Biological Engineering Academy Figure 3. Antioxidant activity of EGCG detected by a scavenging DPPH radical assay. EGCG signifi cantly increased the scavenged DPPH radicals in a concentration-dependent manner. *p 0.05 according to one-way ANOVA. Data are presented as ± standard error of the mean (SEM), obtained from three independent experiments.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)