JOURNAL OF COSMETIC SCIENCE 48 epithelial–mesenchymal contact and stabilize the BMZ. These include laminins, some integrins, and collagen VII (14,15). They have a crucial role in the maintenance of the hair follicle and the control of its volume. Microinfl ammation is suspected to be a precipitating factor in male pattern alopecia (16), but the concept has yet to be integrated into treatment strategies. Exposure to irritants, pollution, and UV radiation has the potential to turn keratinocytes into mediators of infl ammation (17). Under stress conditions, keratinocytes react by increasing their production of interleukin (IL)-1α, a pro-infl ammatory cytokine. The latter acts on fi broblasts to stimulate their production of IL-8, a cytokine involved in the recruit- ment of neutrophils. Both IL-1α and IL-8 are inducible at the dermal papilla and were found in plucked hair samples of subjects with male pattern alopecia (18) sug- gesting their participation in the pathology (19). Cytokine-driven persistent infl am- mation also activates matrix metalloproteinases involved in tissue remodeling and perifollicular fi brosis (20). Limited treatments are currently available for male pattern alopecia. The most popular are the following: minoxidil (Rogain® McNeil-PPC, Johnson & Johnson, New Bruns- wick, New Jersey, USA.), an over-the-counter vasodilator that is believed to optimize blood supply to the dermal papilla (5) fi nasteride (Propecia® Merck, Whitehouse Station, New Jersey), a drug that acts by inhibiting the enzyme that converts testosterone to DHT (5) and diaminopyrimidine oxide (Aminexil®, l’Oréal, Paris, France), a patented compound that prevents perifollicular fi brosis. Each of these treatments targets one aspect of hair loss and offers a certain level of effi cacy for those who respond. However, for improved results, it may be desirable to simultaneously target several aspects of the problem. The new cosmetic active ingredient (a mixture of clover extract and acetyl tetrapeptide-3), described in this article, represents a new, more integrative approach to hair loss. MATERIAL AND METHODS TEST MATERIAL The test material consisted of a mixture of Trifolium pratense (clover) fl ower extract (total isofl avone ≥98% and biochanin A ≥12%, determined by high-performance liquid chro- matography (HPLC)) and acetyl tetrapeptide-3 (pure peptide obtained by solid phase peptide synthesis, purity ≥90% determined by HPLC). The clover extract fraction is standardized using biochanin A, a phytoestrogen fl avonoid with documented health- promoting activities (20). The tetrapeptide is a biomimetic derived from a signal peptide found in matrix proteins, such as collagen and fi brin, and also in HGF, which is a growth factor fi rst isolated from human plasma (21). The peptide is normally liberated by prote- olysis in the course of tissue damage. Its release and activation stimulates tissue remodel- ing following the initial phase of wound healing. The components of the mixture were tested either together or alone, according to their expected roles in hair care, as could be deduced from the existing literature (20,22). An effect of the biochanin A component was documented on 5-α-reductase activity, while acetyl tetra- peptide-3 was investigated for its infl uence on ECM components, including collagens III and VII, and laminins. The mixture of both components was tested for anti-infl ammatory activity before being clinically tested in humans to evidence effi cacy in reducing hair loss.
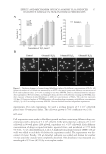
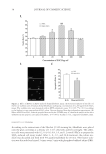
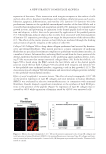
A NEW STRATEGY TO MODULATE ALOPECIA 49 5-α-REDUCTASE ACTIVITY The enzyme 5-α-reductase catalyzes the conversion of testosterone to DHT. The effects of biochanin A on 5-α-reductase activity were studied in intact cells expressing type 1 or type 2 isoforms of the enzyme and compared to that of epigallocatechin-3-gallate (EGCG) from green tea. EGCG is a known in vitro inhibitor of 5-α-reductase (23). In this assay, radio-labeled testosterone served as substrate. The activity of biochanin A on 5-α-reductase activity was shown by Hiipakka et al. (24). Briefl y, 5-α-reductase-expressing cells were plated at 50,000 per well in a 24-well plate in specifi c medium for 18 h at 37°C. The medium was then changed to 0.5 ml of serum- free medium and 5 μl of biochanin A (100 μM) or EGCG (100 μM) was added and kept for 1 h at 37°C before the addition of 14C-testosterone, at a fi nal concentration of 1.5 μM. Cells were then incubated for an additional 3 h and radioactive steroids were extracted with ethyl acetate. The amounts of labeled testosterone and DHT in extracts were next determined by thin layer chromatography, as a measure of 5-α-reductase activity (for more details, see Ref. 24). IMMUNOFLUORESCENT LABELING OF COLLAGEN III AND LAMININS The effect of acetyl tetrapeptide-3 on the expression of different ECM proteins (collagen III and laminins) was evaluated by selective immunofl uorescence in comparison with un- treated fi broblasts. For this experiment, 3 × 104 human fi broblasts (MRC5 from ATCC CCL) were incubated in Dulbecco’s modified Eagle’s medium (DMEM) (Eurobio Labora- tories, Courtaboeuf, France) containing 10% fetal calf serum (FCS) and supplemented with 1% penicillin/streptomycin. Cells were maintained in a humidifi ed incubator at 37°C with 5% CO2 atmosphere to reach confl uence. Cells were then incubated in the presence or absence of acetyl tetrapeptide-3 (10−7 M, equivalent to 0.05 ppm) for 3 days. These cells were rinsed with phosphate-buffered saline (PBS) and fi xed on slides using methanol (for 10 min at −20°C) followed by acetone fi xation (for 10 min, at 4°C). The slides were then dried at room temperature and rinsed with PBS at a pH of 7.6 for 10 min. The presence of collagen III and laminins in cells was detected by incubating the slides with specifi c antibodies diluted at 1/50e overnight at 4°C, that is, type III anti-collagen (rabbit, Rockland, Gilbertville, PA) and anti-laminin (rabbit, Sigma, St. Louis, MO), respectively. Detection of type III anti-collagen and anti-laminin antibodies was done using a goat anti-rabbit IgM + IgG rhodamine (TRITC) conjugate diluted at 1/100e (Southern Biotech, Birmingham, AL). The corresponding fl uorescent signal was moni- tored using confocal microscopy (Axioplan and Zeiss LSM510 Oberkochen, Germany), allowing for semiquantitative evaluation. IMMUNOHISTOLOGICAL LABELING OF COLLAGEN VII The effect of acetyl tetrapeptide-3 on the expression of collagen VII, a major constituent of anchoring fi brils found in the middle part of the follicular BMZ and around the hair papilla was evaluated using immunohistological techniques. As the junction around the anagen hair follicle and its adjacent connective tissue is similar in terms of composition
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)